Main Model
Anterior : Mitrochondrium
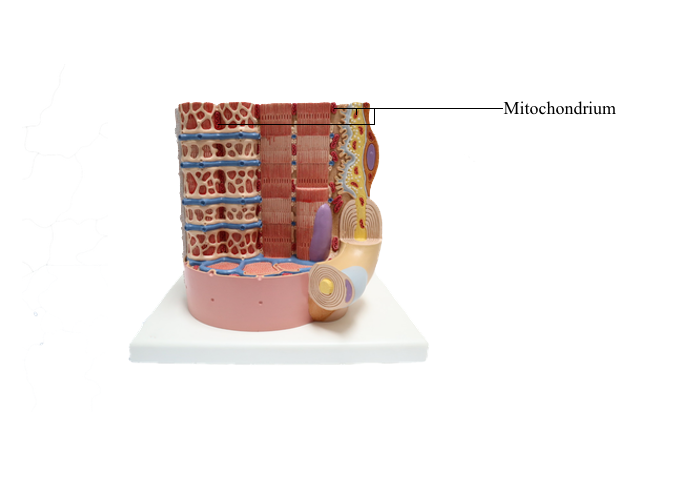
Mitochondria
The mitochondrion (Greek mito, thread; chondrion,
granule) is a highly compartmentalized organelle. The
primary function of mitochondria is to house the
enzymatic machinery for oxidative phosphorylation
resulting in the production of adenosine triphosphate
(ATP) and the release of energy from the metabolism
of molecules.
A mitochondrion consists of an outer mitochondrial membrane and an inner mitochondrial membrane creating an intermembrane space between them. The inner mitochondrial membrane surrounds a large compartment called the
matrix. The matrix is partitioned by infoldings of
the inner mitochondrial membrane known as cristae.
Cristae amplify the inner mitochondrial membrane
on which ATP synthesis takes place.
Mitochondria contain DNA and RNA, including
ribosomes to synthesize some of their own proteins
in the matrix. Only 1% of mitochondrial proteins
are encoded by mitochondrial DNA. Most of mitochondrial proteins are encoded by nuclear genes,
synthesized in cytosol ribosomes and imported into
mitochondria by targeting signals that are recognized
by the translocase of the outer mitochondrial membrane complex (TOM) on the outer mitochondrial
membrane. TOM is the most common entry route
of imported mitochondrial proteins. Targeting polypeptide signals and chaperones (Hsp60 and Hsp70) enable proteins to reach the matrix.
The outer mitochondrial membrane is permeable. It contains porins, proteins that form aqueous
channels permeable to water-soluble molecules with
a reduced molecular mass (less than 5 kd), such as
sugars, amino acids and ions. The inner mitochondrial membrane is impermeable to the passage of
ions and small molecules.
The inner mitochondrial membrane is the site of
electron-transport and proton (H+) pumping and
contains the ATP synthase. Most of the proteins
embedded in the inner mitochondrial membrane
are components of the electron-transport chain,
involved in oxidative phosphorylation.
The mechanism of ATP synthesis is called oxidative phosphorylation. It consists in the addition of
a phosphate group to adenosine diphosphate (ADP)
to form ATP and the utilization of O2. It is also
called chemiosmotic because it involves a chemical
component (the synthesis of ATP) and an osmotic
component (the electron-transport and H+ pumping process).
The mitochondrial matrix contains pyruvate (derived from carbohydrates) and fatty acids (derived from fat). These two small molecules are selectively
transported across the inner mitochondrial membrane and then converted to acetyl coenzyme A
(acetyl CoA) in the matrix.
The citric acid cycle converts acetyl CoA to CO2(released from the cell as waste metabolic product)
and high-energy electrons, carried by nicotinamide
adenine dinucleotide (NADH) - and flavin adenine
dinucleotide (FADH2) - activated carrier molecules.
NADH and FADH2 donate high-energy electrons
to the electron-transport chain lodged in the inner
mitochondrial membrane and become oxidized to
NAD+ and FAD. The electrons travel rapidly along
the transport chain to O2 to form water (H2O).
As the high-energy electrons travel along the
electron-transport chain, energy is released by proton pumps as H+ across the inner mitochondrial
membrane into the intermembrane space. The H+ gradient then drives the synthesis of ATP.
Note that:
1. The inner mitochondrial membrane converts
the energy derived from the high-energy electrons
of NADH into a different type of energy: the high-energy phosphate bond of ATP.
2. The electron-transport chain (or respiratory
chain) contributes to the consumption of O2 as a phosphate group is added to ADP to form ATP.
The components of the electron-transport chain
are present in many copies embedded in the lipid
bilayer of the inner mitochondrial membrane. They
are grouped into three large respiratory enzyme
complexes in the receiving order of electrons:
1. The NADH dehydrogenase complex.
2. The cytochrome b-c1 complex.
3. The cytochrome oxidase complex.
Each complex is a system that pumps H+ across the
inner mitochondrial membrane into the intermembrane space as electrons travel through the complex.
If this mechanism did not exist, the energy released
during electron transfer would produce heat.
Cyanide and azide are poisons that bind to cytochrome oxidase complexes to stop electron transport,
thereby blocking ATP production.
Cytochrome c is a small protein that shuttles
electrons between the cytochrome b-c1 complex and
the cytochrome oxidase complex.
When the cytochrome oxidase complex receives
electrons from cytochrome c, it becomes oxidized
and donates electrons to O2 to form H2O. Four
electrons from cytochrome c and four H+ from the
aqueous environment are added to each molecule of
O2 to form 2H2O.
The H+ gradient across the inner mitochondrial
membrane is used to steer ATP synthesis. ATP synthase is a large enzyme embedded in the inner
mitochondrial membrane involved in ATP synthesis.
H+ flow back across the inner mitochondrial membrane down the electrochemical gradient through
a hydrophilic route within ATP synthase to drive
the reaction between ADP and Pi to produce ATP.
This reaction takes place in the enzymatic
component of ATP synthase projecting into the mitochondrial matrix like a lollipop head. About
100 molecules of ATP are produced per second. About three H+ cross the ATP synthase to form
each molecule of ATP. ADP molecules produced by
ATP hydrolysis in the cytosol are drawn back into
mitochondria for recharging to ATP. ATP molecules
produced in the mitochondrial matrix are released
into the cytosol for their use.
Mitochondria participate in apoptosis, steroidogenesis, and thermogenesis
Mitochondria participate in three significant functions:
1. Programmed cell death or apoptosis.
2. Steroidogenesis (production of steroid hormones).
3. Thermogenesis.
Concerning apoptosis, mitochondria contain
procaspases-2, -3, and -9 (precursors of proteolytic
enzymes), apoptosis initiation factor (AIF), and cytochrome c. The release of these proteins in the cytosol
initiates apoptosis.
With regard to steroidogenesis, mitochondrial
membranes contain enzymes involved in the synthesis
of the steroids aldosterone, cortisol, and androgens.
Concerning thermogenesis, most of the energy from oxidation is dissipated as heat rather than converted to ATP. Uncoupling proteins (UCPs),
members of the superfamily of mitochondrial anion-carrier proteins present in the mitochondrial inner
membrane, mediate the regulated discharge of H+ (called proton leak), resulting in the release of heat.
Proton leak across the mitochondrial inner membrane
is mediated by UCP-1.
UCP-1 is present in the mitochondrial inner
membrane of brown adipocytes. Its role is to mediate regulated thermogenesis in response to cold
exposure.
Clinical significance: Mitochondrial maternal
inheritance
Mitochondrial DNA (mtDNA) is transmitted by
the mother (maternal inheritance). Both males and
females can be affected by mitochondrial diseases, but
males seem unable to transmit the disorder to the offspring. Maternal inheritance of mtDNA is regarded
as an evolutionary advantageous event because of
the potential damage of mtDNA by reactive oxygen
species (ROS) involved in fertilization.
Motile sperm reaching the oviduct for fertilization
eliminate their mtDNA before fertilization, leaving
vacuolar mitochondria. Yet, residual mtDNA in the
fertilizing sperm can be unevenly distributed in the
zygote during early embryo development. Consequently, paternal mtDNA inheritance effects cannot
be disregarded.
Myoclonic epilepsy with ragged red fibers (MERRF)
is characterized by generalized muscle weakness,
loss of coordination (ataxia), and multiple seizures.
The major complications are respiratory and cardiac
failure because the respiratory and cardiac muscles
are affected. Muscle cells and neurons are the most
affected because of their need for significant amounts
of ATP to function.
Histologic preparations of muscle biopsies of individuals with MERRF display a peripheral red-stained
material corresponding to aggregates of abnormal
mitochondria, giving a ragged appearance to red
muscle fibers. MERRF is caused by a point mutation
in a mitochondrial DNA gene encoding tRNA for
lysine. An abnormal tRNA causes a deficiency in the
synthesis of proteins required for electron transport
and ATP production.
Three maternally inherited mitochondrial diseases
affect males more severely than females:
1. About 85% of individuals affected by Leber's
hereditary optic neuropathy (LHON) are male. The disease is confined to the eye. Individuals suffer a sudden loss of vision in the second and third
decades of life.
2. Pearson marrow-pancreas syndrome (anemia
and mitochondrial myopathy observed in childhood).
3. Male infertility. Almost all the energy for sperm
motility derives from mitochondria.