Main Model
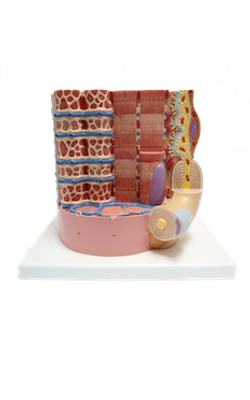
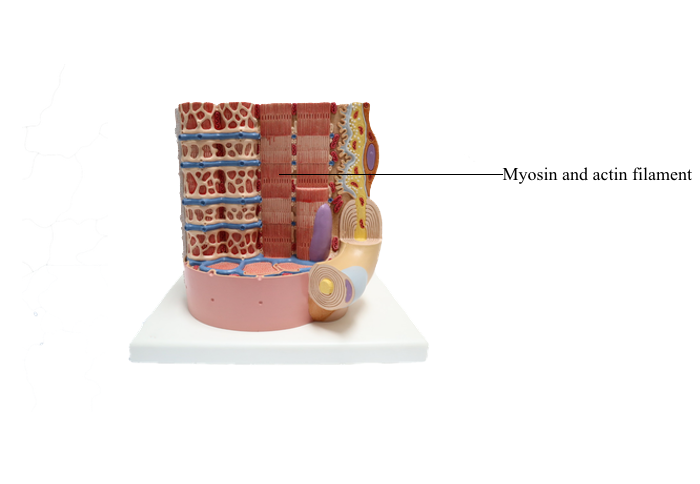
Anterior : Myosin and actin filament
Cytoskeleton
Cytoskeleton is a three-dimensional network of
proteins distributed throughout the cytoplasm of
eukaryotic cells.
The cytoskeleton has roles in:
1. Cell movement (crawling of blood cells along
blood-vessel walls, migration of fibroblasts during
wound healing, and movement of cells during embryonic development)
2. Support and strength for the cell
3. Phagocytosis
4. Cytokinesis
5. Cell-cell and cell–extracellular matrix adherence
6. Changes in cell shape
The components of the cytoskeleton were originally identified by electron microscopy. These early
studies described a system of cytoplasmic “cables” that
fell into three size groups, as follows:
1. Microfilaments (7 nm thick)
2. Intermediate filaments (10 nm thick)
3. Microtubules (25 nm in diameter)
Biochemical studies, involving the extraction of
cytoskeletal proteins from cells with detergents and
salts and in vitro translation of specific mRNA,
showed that each class of filaments has a unique protein organization. When cytoskeletal proteins were
purified, they were used as antigens for the production of antibodies. Antibodies are used as tools for
the localization of the various cytoskeletal proteins
in the cell. The immunocytochemical localization of cytoskeletal proteins (Figure 1-24) and cell treatment with various chemical agents disrupting the
normal organization of the cytoskeleton have been
instrumental in understanding the organization and
function of the cytoskeleton.
Microfilaments
The main component of microfilaments is actin.
Actin filaments are composed of globular monomers
(G-actin, 42 kd), which polymerize to form long
helical filaments intertwined in a helix (F-actin).
Actin is a versatile and abundant cytoskeletal
component forming static and contractile bundles
and filamentous networks specified by actin-binding
proteins and their distinctive location and function
in a cell. F-actin bundles are present in the microvilli
of the intestinal (Figure 1-25) and renal epithelial
cells (brush border) and the stereocilia from the hair
cells of the inner ear.
We have already seen that the intracellular portion
of the cell adhesion molecules cadherins and integrin
`1
interacts with F-actin through linker proteins
(see Figures 1-8 and 1-11). As discussed in Chapter
6, Blood and Hematopoiesis, actin, together with
spectrin, forms a filamentous network on the inner
face of the red blood cell membrane that is crucial
for maintaining the shape and integrity of red blood
cells. Spectrin is a tetramer consisting of two distinct
polypeptide chains (_ and `).
Actin filaments are polar. Growth of actin filaments may occur at both ends; however, one end
(the “barbed end” or plus end) grows faster than the
other end (the “pointed end” or minus end). The
names correspond to the arrowhead appearance of
myosin head bound at an angle to actin.
Actin filaments can branch in the leading edge
(lamellipodia) of cells involved in either motility or
interaction with other cell types. F-actin branching
is initiated from the side of a preexisting actin filament by Arp2/3 (for actin-related protein), an actin
nucleating complex of seven proteins (Figure 1-26).
Formin regulates the assembly of unbranched actin
in cell protrusions such as the intestinal microvilli
(see Figure 1-25).
Actin monomers have a binding site for adenosine triphosphate (ATP), which is hydrolyzed to
adenosine diphosphate (ADP) as polymerization
proceeds. Actin polymerization is ATP-dependent
(see Box 1-E).
The kinetics of actin polymerization involves a
mechanism known as treadmilling: G-actin monomers assembled at one end of the filament concurrently disassemble at the other end (see Figure 1-26).
Four types of proteins control treadmilling (see Figure
1-26), as follows:
1. Thymosin sequesters pools of G-actin monomers within cells.
2. Profilin suppresses nucleation of G-actin and
promotes F-actin growth at the barbed end. Profilin
can favor the assembly of monomeric G-actin into
filaments by facilitating the exchange of bound
ADP for ATP. Only ATP-actin monomers can be
assembled into filaments.
3. Cofilin (also known as actin depolymerizing factor) triggers depolymerization of ADP-bound actin
at the pointed end. Similar to profilin and thymosin,
cofilin forms a dimeric complex with G-actin.
4. Gelsolin has a dual role: it is a capping protein
and prevents the loss and addition of actin monomers, and it is a severing protein. In the presence of
Ca2+, gelsolin fragments actin filaments and remains
bound to the barbed end, forming a cap that prevents
further filament growth.
Figure 1-23. Summary of cell junctions and cell adhesion molecules
In the core of the intestinal microvilli, the assembly of G-actin monomers into filaments and the
organization of these filaments into thick bundles are
controlled by various types of actin-binding or actinrelated proteins. A bundle of parallel nonbranching
actin filaments, forming the core of the microvillus,
is held together by actin-linking proteins, villin and
fimbrin. Side arms of myosin-I and the Ca2+-binding
protein calmodulin anchor the bundle to the plasma
membrane (see Figure 1-25).
Arp2/3 and additional regulatory proteins form a
nucleation complex for the assembly of branching
actin filaments.
Branching actin filaments assemble at the leading edge of a cell during cell motility. In microvilli,
formins (proteins with highly conserved formin homology domains, FH1 and FH2), instead of the
Arp2/3 complex, seem to regulate the elongation of
nonbranching actin filaments, while remaining attached to the barbed end (see Box 1-E).
Formins are located at the tip of the microvillus,
the cap region (see Figure 1-25).
Male patients with defects in proteins that activate
the Arp2/3 complex, in particular a protein of the
Wiskott-Aldrich syndrome protein (WASP) family,
display recurrent respiratory infections because of hereditary immunodeficiency, thrombocytopenia (low
platelet count) present from birth on and eczema of
the skin after the first month of life (see Box 1-F).
The mutation is inherited from the mother, a healthy
carrier of the defective gene.
Microvilli and stereocilia are comparable structures, although they differ in length and the number
of actin filaments:
1. Intestinal microvilli are 1 to 2 +m long, 0.1 +m
wide, and consist of 20 to 30 bundled actin filaments.
2. Stereocilia in hair cells of the inner ear have a
tapered shape at their base, the length range is 1.5 to
5.5 +m, and each actin bundle contains up to 900
actin filaments.
Hair cells are extremely sensitive to mechanical
displacement, and a slight movement of the stereocilium is amplified into changes in electric potential
transmitted to the brain.
We study hair cells of the inner ear in Chapter 9,
Sensory Organs: Vision and Hearing.
Microtubules
Microtubules are composed of tubulin dimers (Figure 1-27; see Box 1-G). Each tubulin dimer consists of two tightly bound tubulin molecules: _-tubulin
and `-tubulin. Tubulin subunits are arranged in
longitudinal rows called protofilaments. Thirteen
protofilaments associate side by side with each other
to form a cylinder of microtubules with a hollow core.
The diameter of a microtubule is 25 nm.
Similar to actin filaments, microtubules are structurally polarized. Microtubules have a plus end,
which grows more rapidly than the minus end (see
Figure 1-27).
In contrast to actin filaments, most individual
microtubules seem to undergo alternate phases of
slow growth and rapid depolymerization. This
process, called dynamic instability, consists of three
major steps:
1. A polymerization phase, in which GTP-tubulin subunits add to the plus end of the microtubule and
a GTP cap is assembled to facilitate further growth.
2. The release of hydrolyzed phosphate (Pi) from
tubulin-bound GTP.
3. A depolymerization phase, in which GDP- tubulin subunits are released from the minus end at
a fast rate.
The polymerization-to-depolymerization transition
frequency is known as catastrophe; the depolymerization-to-polymerization transition frequency is known
as rescue.
The stability of microtubules can be modified by
microtubule-associated proteins (MAPs). MAPs are
classified into two groups:
1. Classical MAPs, such as MAP1A, MAP1B,
MAP2, and tau.
2. Nonclassical MAPs, including Lis1 and DCX
family members. MAPs stabilize microtubules by
phosphorylation/dephosphorylation.
In Chapter 7, Nervous Tissue, we discuss the significance of tau phosphorylation and dephosphorylation in Alzheimer’s disease. A lack of expression
of Lis1 causes a sever brain developmental disorder
called lissencephaly.
Centrosome
The centrosome, the major microtubule-organizing
center in cells, consist of a pair of centrioles surrounded by the pericentriolar material, an amorphous, electron-dense substance rich in proteins such
as pericentrin and a-tubulin.
The centrosome has four major functions:
1. It nucleates the polymerization of tubulin subunits into microtubules.
2. It organizes microtubules into functional units,
for example, the mitotic spindle.
3. It duplicates once every cell cycle in preparation
for cell division.
4. It gives rise to basal body precursors, the originators of multiple or single cilia.
Centrosome abnormalities, in particular an increase
in their number, are frequent in human tumors and
correlate with advanced tumor grade and metastasis.
Therefore, centrosome amplification has a lethal
effect by preventing cells to assemble normal mitotic spindles but also enhancing the potential of
tumorigenesis.
Centrosomes are part of the mitotic center, which,
together with the mitotic spindle, constitutes the
mitotic (or meiotic) apparatus (Figure 1-28). A
centriole is a small cylinder (0.2 +m wide and 0.4
+m long) composed of nine microtubule triplets
in a helicoid array. In contrast to most cytoplasmic
microtubules, which display dynamic instability, the
centriolar microtubules are very stable.
During interphase, centrioles are oriented at right
angles to each other. Before mitosis, centrioles replicate and form two pairs. During mitosis, each pair
can be found at opposite poles of the cell, where they
direct the formation of the mitotic or meiotic spindle.
There are three types of microtubules extending from the centrosomes:
1. Radiating or astral microtubules, anchoring
each centrosome to the plasma membrane.
2. Kinetochore microtubules, attaching the chromosome-associated kinetochore to the centrosomes.
3. Polar microtubules, extending from the two
poles of the spindle where opposite centrosomes are
located (see Figure 1-28).
Kinetochores are formed by several proteins assembled on centromeric DNA during mitosis and
meiosis. The centromere is the chromosomal site
where the kinetochore assembles. If kinetochores fail
to assemble, chromosomes cannot segregate properly
(see Box 1-H).
The pericentriolar material contains the a-tubulin
ring complex and numerous proteins, including peri-centrin. Each a-tubulin ring complex is the nucleation site or template for the assembly and growth of
one microtubule. The centrioles do not have a direct
role in the nucleation of microtubules in the centrosome. Tubulin dimers associate to the a-tubulin ring
by the _-tubulin subunit. Consequently, the minus
end of each microtubule points to the centrosome;
the plus end, the growing end, is oriented outward,
free in the cytoplasm.
The axoneme of cilia and flagella
Early in this chapter, we indicate that centrosomes
give rise to precursor basal bodies, which are the
outgrowth origin of cilia (see Figure 1-6) and flagella.
Motile cilia and flagella are cytoplasmic extensions containing a core of microtubules, the axoneme (Figure 1-29). The axoneme consists of nine peripheral
microtubule doublets surrounding a central pair of
microtubules. This arrangement is known as the 9 + 2
configuration.
Each peripheral doublet consists of a complete
microtubule (called an A tubule, with 13 protofilaments), sharing its wall with a second, partially
completed microtubule (called a B tubule, with 10
to 11 protofilaments). Extending inward from the A
tubule are radial spokes that insert into an amorphous
inner sheath surrounding the central microtubule
pair. Adjacent peripheral doublets are linked by the
protein nexin (see Box 1-I).
Projecting from the sides of the A tubule are sets
of protein arms: the inner and outer arms of dynein,
a microtubule-associated adenosine triphosphatase
(ATPase). In the presence of ATP, the sliding of
peripheral doublets relative to each other bends cilia
and flagella. Sliding and bending of microtubules are
the basic events of their motility.
Ciliopathies can occur when defects occur during:
1. The multiplication and docking of the centrosome-derived precursor basal bodies. An example is
the enhanced expression of the protein CP110 that
prevents the attachment of basal bodies to the plasma
membrane, leading to primary ciliary dyskinesia.
2. The transport of proteins during the assembly
of cilia and flagella, resulting in the Bardet-Biedl
syndrome (see Box 1-J; see Figure 1-6).
Clinical significance: Microtubule-targeted drugs.
Sterility
Two groups of antimitotic drugs act on microtubules:
1. Microtubule-destabilizing agents, which inhibit
microtubule polymerization.
2. Microtubule-stabilizing agents, which affect
microtubule function by suppressing dynamic instability.
The first group includes colchicine, colcemid,
vincristine, and vinblastine, which bind to tubulin
and inhibit microtubule polymerization, blocking
mitosis. Colchicine is used clinically in the treatment
of gout. Vincristine and vinblastine, from Vinca alkaloids isolated from the leaves of the periwinkle plant,
have been successfully used in childhood hematologic
malignancies (leukemias). Neurotoxicity, resulting
from the disruption of the microtubule-dependent
axonal flow (loss of microtubules and binding of motor proteins to microtubules), and myelosuppression
are two side effects of microtubule-targeted drugs.
The second group includes taxol (isolated from
the bark of the yew tree) with an opposite effect: It
stabilizes microtubules instead of inhibiting their assembly (Figure 1-30). Paclitaxel (taxol) has been used
widely to treat breast and ovarian cancers. Similar to
Vinca alkaloids, its main side effects are neurotoxicity and suppression of hematopoiesis.
Kartagener’s syndrome is an autosomal recessive ciliary dyskinesia frequently associated with
bronchiectasis (permanent dilation of bronchi and
bronchioles) and sterility in men.
Kartagener’s syndrome is the result of structural
abnormalities in the axoneme (defective or absent
dynein) that prevent mucociliary clearance in the
airways (leading to persistent infections) and reduce
sperm motility and egg transport in the oviduct
(leading to sterility).
Microtubules: Cargo transport and motor proteins
The transport of vesicles and nonvesicle cargos occurs
along microtubules and F-actin.
Specific molecular motors associate to microtubules
and F-actin to mobilize cargos to specific intracellular sites.
Microtubule-based molecular motors include
kinesin and cytoplasmic dynein for the long-range
transport of cargos.
F-actin–based molecular motors include unconventional myosin Va and VIIa for the short-range
transport of cargos. We discuss additional aspects of
the F-actin–based cargo transport mechanism during the transport of melanosomes in Chapter 11,
Integumentary System.
Three examples of microtubule-based cargo
transport in mammalian systems are as follows (see
Box 1-K):
1. Axonemal transport, including flagella (intraflagellar transport) and cilia (intraciliary transport)
(Figure 1-31). During axonemal transport, particles
are mobilized by kinesin and cytoplasmic dynein
along the microtubule doublets of the axoneme.
Defective axonemal transport results in the abnormal assembly of cilia and flagella, including polycystic
kidney disease, retinal degeneration, respiratory ciliary dysfunction, and lack of sperm tail development.
As indicated before (see Box 1-J), the Bardet-Biedl
syndrome is a disorder caused by basal body/ciliary
dysfunction secondary to a defective microtubulebased transport function.
2. Axonal transport, along the axon of neurons
(see Figure 1-31).
3. Intramanchette transport, along microtubules of the manchette, a transient structure assembled
during the elongation of the spermatid head (see
Chapter 20, Spermatogenesis).
Microtubules: Axonal transport
Axons are cytoplasmic extensions of neurons responsible for the conduction of neuronal impulses. Membrane-bound vesicles containing neurotransmitters
produced in the cell body of the neuron travel to the
terminal portion of the axon, where the content of
the vesicle is released at the synapse.
Bundles of microtubules form tracks within the
axon to carry these vesicles. Vesicles are transported
by two motor proteins (see Figure 1-31):
1. Kinesin
2. Cytoplasmic dynein
Kinesins and cytoplasmic dyneins participate in
two types of intracellular transport movements:
1. Saltatory movement, defined by the continuous
and random movement of mitochondria and vesicles.
2. Axonal transport, a more direct intracellular
movement of membrane-bound structures.
Kinesins and cytoplasmic dyneins have two
ATP-binding heads and a tail. Energy derives from
continuous ATP hydrolysis by ATPases present in the
heads. The head domains interact with microtubules,
and the tail binds to specific receptor binding sites on the surface of vesicles and organelles.
Kinesin uses energy from ATP hydrolysis to move
vesicles from the cell body of the neuron toward the
end portion of the axon (anterograde transport).
Cytoplasmic dynein also uses ATP to move vesicles in
the opposite direction (retrograde transport).
Myosin family of proteins
Members of the myosin family of proteins bind and
hydrolyze ATP to provide energy for their movement
along actin filaments from the pointed (minus) end
to the barbed (plus) end. Myosin I and myosin II
are the predominant members of the myosin family
(Figure 1-32; see Box 1-L).
Myosin I, regarded as an unconventional myosin, is
found in all cell types and has only one head domain
and a tail. The head is associated with a single light
chain. The head interacts with actin filaments and
contains ATPase, which enables myosin I to move
along the filaments by binding, detaching, and rebinding. The tail binds to vesicles or organelles. When
myosin I moves along an actin filament, the vesicle
or organelle is transported. Myosin I molecules are
smaller than myosin II molecules, lack a long tail, and
do not form dimers.
Myosin II, a conventional myosin, is present in
muscle and nonmuscle cells. Myosin II consists of a
pair of identical molecules. Each molecule consists
of an ATPase-containing head domain and a long
rodlike tail. The tails of the dimer link to each other
along their entire length to form a two-stranded
coiled rod. The tail of myosin II self-assembles into
dimers, tetramers, and a bipolar filament with the
heads pointing away from the midline.
The two heads, linked together but pointing in
opposite directions, bind to adjacent actin filaments
of opposite polarity. Each myosin head bound to Factin moves toward the barbed (positive) end. Consequently, the two actin filaments are moved against
each other, and contraction occurs (see Figure 1-32).
Heads and tails of myosin II can be cleaved by
enzymes (trypsin or papain) into light meromyosin
(LMM) and heavy meromyosin (HMM). LMM
forms filaments, but lacks ATPase activity and does
not bind to actin. HMM binds to actin, is capable of
ATP hydrolysis, and does not form filaments. HMM
is responsible for generating force during muscle
contraction. HMM can be cleaved further into two
subfragments called S1. Each S1 fragment contains
ATPase and light chains and binds actin.
Myosin V, an unconventional myosin, is doubleheaded with a coiled double tail. The head region
binds to F-actin; the distal globular ends of the tails
bind to Rab27a, a receptor on vesicle membranes.
Myosin Va mediates vesicular transport along F-actin
tracks. A specific example is the transport of melanosomes from melanocytes to keratinocytes, first along
microtubules and later along F-actin (see Chapter 11,
Integumentary System).
Mutations in the Rab27a and myosin Va genes
disrupt the F-actin transport of melanosomes. An
example in humans is Griscelli syndrome, a rare autosomal recessive disorder characterized by pigment
dilution of the hair caused by defects in melanosome
transport and associated with disrupted T cell cytotoxic activity and neurologic complications.
Figure 1-33 summarizes the relevant structural and
functional characteristics of motor proteins.
Myosin light-chain kinase
The self-assembly of myosin II and interaction
with actin filaments in nonmuscle cells takes place
in certain sites according to functional needs. These
events are controlled by the enzyme myosin lightchain kinase (MLCK), which phosphorylates one
of the myosin light chains (called the regulatory
light chain) present on the myosin head. The activity
of MLCK is regulated by the Ca2+-binding protein
calmodulin (Figure 1-34).
MLCK has a catalytic domain and a regulatory
domain. When calmodulin and Ca2+ bind to the
regulatory domain, the catalytic activity of the kinase
is released. The MLCK–calmodulin–Ca2+ complex
catalyzes the transfer of a phosphate group from ATP
to the myosin light chain, and myosin cycles along
F-actin to generate force and muscle contraction.
Phosphorylation of one of the myosin light chains
results in two effects:
1. It exposes the actin-binding site on the myosin
head. This step is essential for an interaction of the
myosin head with the F-actin bundle.
2. It releases the myosin tail from its sticky attachment site near the myosin head. This step also
is critical because only myosin II stretched tails can
self-assemble and generate bipolar filaments, a requirement for muscle contraction (see Figure 1-33).
In smooth muscle cells, a phosphatase removes the
phosphate group from myosin light chains. Skeletal
muscle contraction does not require phosphorylation
of the myosin light chains. We discuss additional details of muscle contraction when we study the muscle
tissue (see Chapter 7, Muscle Tissue).
Intermediate filaments
Intermediate filaments (Figure 1-35) represent a
heterogeneous group of structures so named because
their diameter (10 nm) is intermediate between
those of microtubules (25 nm) and microfilaments
(7 nm). Intermediate filaments are the most stable
cytoskeletal structures.
Detergent and salt treatments extract microfilament
and microtubule components and leave intermediate
filaments insoluble. The structure of the intermediate filament does not fluctuate between assembly
and disassembly states similar to microtubules and
microfilaments. Note that in contrast to microtubules and actin filaments, which are assembled
from globular proteins with nucleotide-binding and
hydrolyzing activity, intermediate filaments consists
of filamentous monomers lacking enzymatic activity.
In contrast to actin and tubulin, the assembly and
disassembly of intermediate filament monomers are
regulated by phosphorylation and dephosphorylation, respectively.
Intermediate filament protein monomers consist of three domains (see Figure 1-35): A central _-helical
rod domain is flanked by a nonhelical N-terminal
head domain and a C-terminal tail domain.
The assembly of intermediate filaments occurs in
four steps:
1. A pair of filamentous monomers of variable
length and amino acid sequence of the head and tail
domains, form a parallel dimer through their central
rod domain coiled around each other.
2. A tetrameric unit is then assembled by two
antiparallel half staggered coiled dimers. Therefore,
in contrast to microtubules and actin filaments, the
antiparallel alignment of the initial tetramers determines a lack of structural polarity of intermediate
filament (absence of plus and minus ends). One end of an intermediate filament cannot be distinguished
from another. If molecular motors associate to an
intermediate filament, they would find it difficult to
identify one direction from another.
3. Eight tetramers associate laterally to form a 16
nm-thick unit length filament (ULF).
4. Individual ULFs join end-to-end to form a short
filaments that continue growing longitudinally by
annealing to other ULFs and existing intermediate
filaments. The elongation of the filament is followed
by internal compaction to achieve the 10 nm-thick
intermediate filament.
The tight association of dimers, tetramers and
ULFs provide intermediate filaments with high tensile
strength and resistance to stretching, compression,
twisting and bending forces.
Intermediate filaments provide structural strength
or scaffolding for the attachment of other structures.
Intermediate filaments form extensive cytoplasmic
networks extending from cage-like perinuclear arrangements to the cell surface.
Intermediate filaments of different molecular
classes are characteristic of particular tissues or states
of differentiation (for example, in the epidermis of
skin). Five major types of intermediate filament proteins have been identified on the basis of sequence
similarities in the _-helical rod domain. They are
referred to as types I through V (see Box 1-M).
About 50 intermediate filament proteins have been
reported so far.
Type I (acidic keratins) and type II (neutral
to basic keratins). This class of proteins forms the
intermediate filament cytoskeleton of epithelial cells
(called cytokeratins to distinguish them from the
keratins of hair and nails). Equal amounts of acidic
(40 to 60 kd) and neutral-basic (50 to 70 kd) cytokeratins combine to form this type of intermediate filament protein. Type I and type II intermediate
filament keratins form tonofilaments associated
with molecules present in the cytoplasmic plaques
of desmosomes and hemidesmosomes (see Figures
1-18 and 1-19). We come back to intermediate filament–binding proteins, such as filaggrins, when we
discuss the differentiation of keratinocytes in the
epidermis of the skin (Chapter 11, Integumentary
System), and plectin, when we analyze the cytoskeletal
protective network of skeletal muscle cells (Chapter
7, Muscle Tissue).
In the epidermis, the basal cells express keratins
K5 and K14. The upper differentiating cells express
keratins K1 and K10. In some regions of the epidermis, such as in the palmoplantar region, keratin K9 is
found. Mutations in K5 and K14 cause hereditary
blistering skin diseases belonging to the clinical type
epidermolysis bullosa simplex (see later, Clinical
significance: Intermediate filaments and skin blistering diseases).
Type III. This group includes the following intermediate filament proteins:
Vimentin (54 kd) is generally found in cells of
mesenchymal origin.
Desmin (53 kd) is a component of skeletal muscle
cells and is localized to the Z disk of the sarcomere
(see Chapter 7, Muscle Tissue). This intermediate
filament protein keeps individual contractile elements
of the sarcomeres attached to the Z disk and plays a
role in coordinating muscle cell contraction. Desmin is also found in smooth muscle cells.
Glial fibrillary acidic protein (GFAP) (51 kd) is
observed in astrocytes and some Schwann cells (see
Chapter 8, Nervous Tissue).
Peripherin (57 kd) is a component of neurons of
the peripheral nervous system and is coexpressed
with neurofilament proteins (see Chapter 8, Nervous
Tissue).
Type IV. This group includes neurofilaments,
nestin, syncoilin and _-internexin. Neurofilaments
are the main components.
Neurofilaments (NFs) are found in axons and dendrites of neurons. Three types of proteins can
be found in a neurofilament: NF-L (60 to 70 kd),
NF-M (105 to 110 kd), and NF-H (135 to 150 kd),
for low-molecular-weight, middle-molecular-weight,
and high-molecular-weight neurofilaments. Abnormal accumulations of neurofilaments (neurofibrillary
tangles) are a characteristic feature of a number of
neuropathologic conditions.
_-Internexin (66 kd) is found predominantly in
the central nervous system (particularly in the spinal
cord and optic nerve).
Type V. Proteins of this group, the nuclear lamins,
are encoded by three genes: LMNA, LMNB1, and
LMNB2.
Lamin A and lamin C arise from the alternative
splicing of transcripts encoded by the LMNA gene.
The LMNB1 gene encodes lamin B1 expressed in all
somatic cells. The LMNB2 gene encodes lamin B2,
expressed in all somatic cells, and lamin B3, that is
specific for spermatogenic cells.
Nuclear lamins (60 to 75 kd) differ from the other
intermediate filament proteins in that they organize
an orthogonal meshwork, the nuclear lamina, in
association with the inner membrane of the nuclear
envelope.
Lamins provide mechanical support for the nuclear
envelope and bind chromatin. Because of their clinical relevance, we come back to nuclear lamins and
associated proteins when we discuss the organization
of the nuclear envelope.
A group of human diseases, known as laminopathies, are linked to defects in proteins of the nuclear
envelope, including lamins (see Box 1-N). Numerous
laminopathies affect cardiac and skeletal muscle,
adipose tissue (lipodystrophies), and motor and
sensory peripheral nerves.
Two hypotheses concerning the pathogenic mechanism of laminopathies have been considered:
1. The gene expression hypothesis regards lamin A and lamin C as essential for the correct tissue-specific
expression of certain genes.
2. The mechanical stress hypothesis proposes that a
defect in lamin A and lamin C weakens the structural
integrity of the nuclear envelope.
During mitosis, the phosphorylation of lamin
serine residues causes a transient disassembly of the
meshwork, followed by a breakdown of the nuclear
envelope into small fragments. At the end of mitosis, lamins are dephosphorylated, and the lamin
meshwork and the nuclear envelope reorganize. See
the cell nucleus section concerning the mechanism
of phosphorylation and dephosphorylation of lamins
during the cell cycle.
Hemidesmosomes and intermediate filaments
Hemidesmosomes are specialized junctions observed
in basal cells of the stratified squamous epithelium
attaching to the basement membrane (Figure 1-36).
Inside the cell, the proteins BPAG1 (for bullous
pemphigoid antigen 1) and plectin (members of the
plakin family of cross-linker proteins) are associated
to intermediate filaments (also called tonofilaments).
Plectin connects intermediate filaments to the integrin subunit `4
.
On the extracellular side, integrin _6
`4
, BPAG2
(for bullous pemphigoid antigen 2) and laminin
5, a protein present in specialized structures called
anchoring filaments, link hemidesmosomes to the
basal lamina.
The plakin-related protein BPAG1 associates to
BPAG2, a transmembrane protein with an extracellular collagenous domain.
Putting all things together, BPAG1 constitutes a
bridge between the transmembrane protein BPAG2
and intermediate filaments. If this bridge is disrupted,
as in bullous pemphigoid, the epidermis becomes detached from the basal lamina anchoring sites. BPAG1
and BPAG2 were discovered in patients with bullous
pemphigoid, an autoimmune disease.
Clinical significance: Skin blistering diseases
Bullous pemphigoid is an autoimmune blistering
disease similar to pemphigus vulgaris (called “pemphigoid”, similar to pemphigus). Blisters or bullae
develop at the epidermis-dermis junction when
circulating immunoglobulin G (IgG) cross-reacts
with bullous pemphigoid antigen 1 or 2. IgG-antigen
complexes lead to the formation of complement complexes (C3, C5b, and C9), which damage the attachment of hemidesmosomes and perturb the synthesis of anchoring proteins by basal cells (Figure 1-37).
The production of local toxins causes the degranulation of mast cells and release of chemotactic
factors attracting eosinophils. Enzymes released by
eosinophils cause blisters or bullae.
Intermediate filaments strengthen the cellular
cytoskeleton. The expression of mutant keratin
genes results in the abnormal assembly of keratin
filaments, which weakens the mechanical strength
of cells and causes inherited skin diseases, as shown
in Figure 1-38:
1. Epidermolysis bullosa simplex (EBS), characterized by skin blisters after minor trauma. EBS is
determined by keratin 5 and 14 mutant genes.
2. Epidermolytic hyperkeratosis (EH), in which
patients have excessive keratinization of the epidermis
owing to mutations of keratin 1 and 10 genes.
3. Epidermolytic plantopalmar keratoderma
(EPPK), a skin disease producing fragmentation of
the epidermis of the palms and soles, caused by a
mutation of the keratin 9 gene.