Main Model
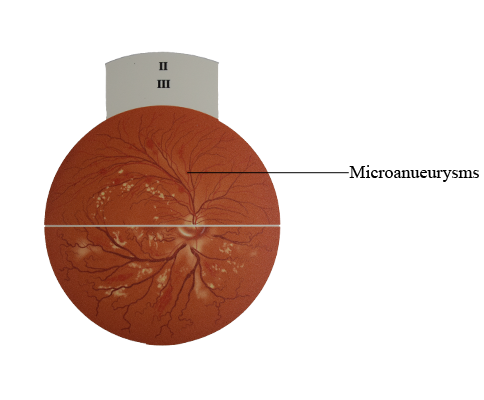
Diabetic retinopathy and Hypertensive retinopathy : Microanueurysms
DIABETIC RETINOPATHY
Introduction
Ophthalmic complications of diabetes
• Common
○ Retinopathy.
○ Iridopathy (minor iris transillumination defects).
○ Unstable refraction.
• Uncommon
○ Recurrent styes.
○ Xanthelasmata.
○ Accelerated senile cataract.
○ Neovascular glaucoma (NVG).
○ Ocular motor nerve palsies.
○ Reduced corneal sensitivity.
• Rare. Papillopathy, pupillary light-near dissociation,
Wolfram syndrome (progressive optic atrophy and multiple
neurological and systemic abnormalities), acute-onset
cataract, rhino-orbital mucormycosis.
Prevalence
The reported prevalence of diabetic retinopathy (DR) in diabetics
varies substantially between studies, even amongst contemporary
populations in the same country, but is probably around 40%. It
is more common in type 1 diabetes than in type 2 and sightthreatening disease is present in up to 10%. Proliferative diabetic retinopathy (PDR) affects 5–10% of the diabetic population; type
1 diabetics are at particular risk, with an incidence of up to 90%
after 30 years.
Risk factors
• Duration of diabetes is the most important risk factor. In
patients diagnosed with diabetes before the age of 30 years,
the incidence of DR after 10 years is 50%, and after 30 years
90%. DR rarely develops within 5 years of the onset of
diabetes or before puberty, but about 5% of type 2 diabetics
have DR at presentation. It appears that duration is a stronger
predictor for proliferative disease than for maculopathy.
• Poor control of diabetes. It has been shown that tight blood
glucose control, particularly when instituted early, can
prevent or delay the development or progression of DR.
However, a sudden improvement in control may be
associated with progression of retinopathy in the near term.
Type 1 diabetic patients appear to obtain greater benefit
from good control than type 2. Raised HbA1c is associated
with an increased risk of proliferative disease.
• Pregnancy is sometimes associated with rapid progression of
DR. Predicating factors include greater pre-pregnancy
severity of retinopathy, poor pre-pregnancy control of
diabetes, control exerted too rapidly during the early stages
of pregnancy, and pre-eclampsia. The risk of progression is
related to the severity of DR in the first trimester. If
substantial DR is present, frequency of review should reflect
individual risk, and can be up to monthly. Diabetic macular
oedema usually resolves spontaneously after pregnancy and
need not be treated if it develops in later pregnancy.
• Hypertension, which is very common in patients with type 2
diabetes, should be rigorously controlled (<140/80 mmHg).
Tight control appears to be particularly beneficial in type 2
diabetics with maculopathy. Cardiovascular disease and
previous stroke are also predictive.
• Nephropathy, if severe, is associated with worsening of DR.
Conversely, treatment of renal disease (e.g. renal
transplantation) may be associated with improvement of
retinopathy and a better response to photocoagulation.
• Other risk factors include hyperlipidaemia, smoking,
cataract surgery, obesity and anaemia.
Pathogenesis
DR is predominantly a microangiopathy in which small blood
vessels are particularly vulnerable to damage from high glucose
levels. Direct hyperglycaemic effects on retinal cells are also likely
to play a role.
Many angiogenic stimulators and inhibitors have been identified; vascular endothelial growth factor (VEGF) appears to be of
particular importance in the former category.
Classification
The classification used in the Early Treatment Diabetic Retinopathy Study (ETDRS – the modified Airlie House classification) is widely used internationally. An abbreviated version is set out in
Table 13.1, in conjunction with management guidelines. The following descriptive categories are also in widespread use in clinical
practice:
• Background diabetic retinopathy (BDR) is characterized by
microaneurysms, dot and blot haemorrhages and exudates.
These are generally the earliest signs of DR, and persist as
more advanced lesions appear.
• Diabetic maculopathy strictly refers to the presence of any
retinopathy at the macula, but is commonly reserved for
significant changes, particularly vision-threatening oedema
and ischaemia.
• Preproliferative diabetic retinopathy (PPDR) manifests
with cotton wool spots, venous changes, intraretinal
microvascular anomalies (IRMA) and often deep retinal
haemorrhages. PPDR indicates progressive retinal ischaemia,
with a heightened risk of progression to retinal
neovascularization.
• PDR is characterized by neovascularization on or within one
disc diameter of the disc (NVD) and/or new vessels
elsewhere (NVE) in the fundus.
• Advanced diabetic eye disease is characterized by tractional
retinal detachment, significant persistent vitreous
haemorrhage and neovascular glaucoma.
Signs
Microaneurysms
Microaneurysms are localized outpouchings, mainly saccular, of
the capillary wall that may form either by focal dilatation of the
capillary wall where pericytes are absent, or by fusion of two arms
of a capillary loop (Fig. 13.2A). Most develop in the inner capillary
plexus (inner nuclear layer), frequently adjacent to areas of capillary non-perfusion (Fig. 13.2B). Loss of pericytes (Fig. 13.2C) may
also lead to endothelial cell proliferation with the formation of
‘cellular’ microaneurysms (Fig. 13.2D). Microaneurysms may leak
plasma constituents into the retina as a result of breakdown in the
blood–retinal barrier, or may thrombose. They tend to be the
earliest sign of DR.
• Signs. Tiny red dots, often initially temporal to the fovea
(Fig. 13.3A); may be indistinguishable clinically from dot
haemorrhages.
• Fluorescein angiography (FA) allows differentiation
between dot haemorrhages and non-thrombosed
microaneurysms. Early frames show tiny hyperfluorescent
dots (Fig. 13.3B), typically more numerous than visible
clinically. Late frames show diffuse hyperfluorescence due to
leakage.
Retinal haemorrhages
• Retinal nerve fibre layer haemorrhages arise from the larger
superficial pre-capillary arterioles (Fig. 13.4A) and assume
their characteristic shape (Fig. 13.4B) because of the
architecture of the retinal nerve fibre layer.
• Intraretinal haemorrhages arise from the venous end of
capillaries and are located in the compact middle layers of
the retina (see Fig. 13.4A) with a resultant red ‘dot/blot’
configuration (Fig. 13.4C).
• Deeper dark round haemorrhages (Fig. 13.4D) represent
haemorrhagic retinal infarcts and are located within the
middle retinal layers (see Fig. 13.4A). The extent of
involvement is a significant marker of the likelihood of
progression to PDR.
Exudates
Exudates, sometimes termed ‘hard’ exudates to distinguish from
the older term for cotton wool spots – ‘soft’ exudates, are caused by chronic localized retinal oedema; they develop at the junction
of normal and oedematous retina. They are composed of lipoprotein and lipid-filled macrophages located mainly within the outer
plexiform layer (Fig. 13.5A). Hyperlipidaemia may increase the
likelihood of exudate formation.
• Signs
○ Waxy yellow lesions (Fig. 13.5B) with relatively distinct
margins arranged in clumps and/or rings at the posterior
pole, often surrounding leaking microaneurysms.
○ With time the number and size tend to increase (Fig.
13.5C), and the fovea may be involved.
○ When leakage ceases, exudates absorb spontaneously over
a period of months, either into healthy surrounding
capillaries or by phagocytosis.
○ Chronic leakage leads to enlargement and the deposition
of crystalline cholesterol (Fig. 13.5D).
• FA will commonly show hypofluorescence only with large
dense exudates, as although background choroidal
fluorescence is masked, retinal capillary fluorescence is
generally preserved overlying the lesions (Fig 13.6).
Diabetic macular oedema (DMO)
Diabetic maculopathy (foveal oedema, exudates or ischaemia) is
the most common cause of visual impairment in diabetic patients,
particularly type 2. Diffuse retinal oedema is caused by extensive
capillary leakage, and localized oedema by focal leakage from
microaneurysms and dilated capillary segments. The fluid is initially located between the outer plexiform and inner nuclear
layers; later it may also involve the inner plexiform and nerve fibre
layers, until eventually the entire thickness of the retina becomes oedematous. With central accumulation of fluid the fovea assumes
a cystoid appearance – cystoid macular oedema (CMO) that is
readily detectable on optical coherence tomography (OCT) (Fig.
13.7A) and assumes a central flower petal pattern on FA (Fig.
13.7B).
• Focal maculopathy: well-circumscribed retinal thickening
associated with complete or incomplete rings of exudates
(Fig. 13.8A). FA shows late, focal hyperfluorescence due
to leakage, usually with good macular perfusion
(Fig. 13.8B).
• Diffuse maculopathy: diffuse retinal thickening, which may
be associated with cystoid changes; there are typically also
scattered microaneurysms and small haemorrhages (Fig.
13.9A). Landmarks may be obscured by oedema, which may
render localization of the fovea impossible. FA shows
mid- and late-phase diffuse hyperfluorescence (Fig. 13.9B),
and demonstrates CMO if present.
Ischaemic maculopathy
• Signs are variable and the macula may look relatively
normal despite reduced visual acuity. In other cases PPDR
may be present.
• FA shows capillary non-perfusion at the fovea (an enlarged
FAZ) and frequently other areas of capillary non-perfusion
(Fig. 13.10) at the posterior pole and periphery.
Clinically significant macular oedema
Clinically significant macular oedema (CSMO) is detected on
clinical examination as defined in the ETDRS (Fig. 13.11):
• Retinal thickening within 500 µm of the centre of the
macula (Fig. 13.11, upper left).
• Exudates within 500 µm of the centre of the macula, if
associated with retinal thickening; the thickening itself may
be outside the 500 µm (Fig. 13.11, upper right).
• Retinal thickening one disc area (1500 µm) or larger, any
part of which is within one disc diameter of the centre of the
macula (Fig. 13.11, lower centre).
Cotton wool spots
Cotton wool spots are composed of accumulations of neuronal
debris within the nerve fibre layer. They result from ischaemic
disruption of nerve axons, the swollen ends of which are known
as cytoid bodies, seen on light microscopy as globular structures
in the nerve fibre layer (Fig. 13.12A). As cotton wool spots heal,
debris is removed by autolysis and phagocytosis.
• Signs. Small fluffy whitish superficial lesions that obscure
underlying blood vessels (Fig. 13.12B and C). They are
clinically evident only in the post-equatorial retina, where
the nerve fibre layer is of sufficient thickness to render them
visible.
• FA shows focal hypofluorescence due to local ischaemia and
blockage of background choroidal fluorescence.
Venous changes
Venous anomalies seen in ischaemia consist of generalized dilatation and tortuosity, looping, beading (focal narrowing and dilatation) and sausage-like segmentation (Fig. 13.13). The extent of the
retinal area exhibiting venous changes correlates well with the
likelihood of developing proliferative disease.
Intraretinal microvascular abnormalities
Intraretinal microvascular abnormalities (IRMA) are arteriolar–
venular shunts that run from retinal arterioles to venules, thus
bypassing the capillary bed and are therefore often seen adjacent
to areas of marked capillary hypoperfusion (Fig. 13.14A).
• Signs. Fine, irregular, red intraretinal lines that run from
arterioles to venules, without crossing major blood vessels
(Fig. 13.14B).
• FA shows focal hyperfluorescence associated with adjacent
areas of capillary closure (‘dropout’) but without leakage.
Arterial changes
Subtle retinal arteriolar dilatation may be an early marker of
ischaemic dysfunction. When significant ischaemia is present
signs include peripheral narrowing, ‘silver wiring’ and obliteration, similar to the late appearance following a branch retinal
artery occlusion.
Proliferative retinopathy
It has been estimated that over one-quarter of the retina must be
non-perfused before PDR develops. Although preretinal new
vessels may arise anywhere in the retina, they are most commonly
seen at the posterior pole. Fibrous tissue, initially fine, gradually
develops in association as vessels increase in size.
• New vessels at the disc (NVD) describes neovascularization
on or within one disc diameter of the optic nerve head (Fig.
13.15).
• New vessels elsewhere (NVE) describes neovascularization
further away from the disc (Fig. 13.16); it may be associated
with fibrosis if long-standing.
• New vessels on the iris (NVI – Fig. 13.17), also known as
rubeosis iridis, carry a high likelihood of progression to
neovascular glaucoma (see Ch. 10).
• FA (see Fig. 13.15C) highlights neovascularization during the
early phases of the angiogram and shows irregular expanding
hyperfluorescence during the later stages due to intense
leakage of dye from neovascular tissue. FA can be used to
confirm the presence of new vessels (NV) if the clinical
diagnosis is in doubt, and also delineates areas of ischaemic
retina that might be selectively targeted for laser treatment.