Main Model

Fundus of stomuch : Serous tunic
Stomach
The stomach extends from the esophagus to the duodenum. The epithelium changes from stratified squamous to a simple columnar type at the gastro-esophageal junction. The muscularis mucosae of the esophagus is continuous with that of the stomach. However, the submucosa does not have a clear demarcation line, and glands from the cardiac portion of the stomach may extend under the stratified squamous epithelium and contact the esophageal cardiac glands.
The function of the stomach is to homogenize and chemically process the swallowed semisolid food. Both the contractions of the muscular wall of the stomach and the acid and enzymes secreted by the gastric mucosa contribute to this function. Once the food is transformed into a thick fluid, it is released gradually into the duodenum.
Four regions are recognized in the stomach:
1. The cardia, a 2- to 3-cm-wide zone surrounding the esophageal opening.
2. The fundus, projecting to the left of the opening of the esophagus.
3. The body, an extensive central region.
4. The pyloric antrum (Greek pyloros, gatekeeper), ending at the gastroduodenal orifice.
Based on the motility characteristics of the stomach, the orad area, consisting of the fundus and the upper part of the body, relaxes during swallowing. The caudad area, consisting of the lower portion of the body and the antrum, participates in the regulation of gastric emptying.
The empty stomach shows gastric mucosal folds, or rugae, covered by gastric pits or foveolae. A gastric mucosal barrier, produced by surface mucous cells, protects the mucosal surface. The surface mucous cells contain apical periodic acid-Schiff (PAS) - positive granules and are linked to each other by apical tight junctions.
Cardia Region
Glands of the cardia region are tubular, with a coiled end and an opening continuous with the gastric pits. A mucus-secreting epithelium lines the cardiac glands.
The Gastric Gland
Gastric glands of the fundus-body region are the major contributors to the gastric juice. About 15 million gastric glands open into 3.5 million gastric pits. From two to seven gastric glands open into a single gastric pit, or foveola.
A gastric gland consists of three regions:
1. The pit, or foveola, lined by surface mucous cells.
2. The neck, containing mucous neck cells, mitotically active stem cells, and parietal cells.
3. The body, representing the major length of the gland. The upper and lower portions of the body contain different proportions of cells lining the gastric gland.
The gastric glands proper house five major cell types:
1. Mucous cells, including the surface mucous cells and the mucous neck cells.
2. Chief cells, also called peptic cells.
3. Parietal cells, also called oxyntic cells.
4. Stem cells.
5. Gastroenteroendocrine cells, called enterochromaffin cells because of their staining affinity for chromic acid salts.
The upper portion of the main body of the gastric gland contains abundant parietal cells. Chief cells and gastroenteroendocrine cells predominate in the lower portion.
Mucous Cells
The gastric mucosa of the fundus-body region has two classes of mucus-producing cells:
1. The surface mucous cells, lining the pits.
2. The mucous neck cells, located at the opening of the gastric gland into the pit.
Both cells produce mucins, glycoproteins with high molecular mass. A mucus layer, containing 95% water and 5% mucins, forms an insoluble gel that attaches to the surface of the gastric mucosa, forming a 100-μm-thick protective mucosal barrier. This protective mucus blanket traps bicarbonate ions and neutralizes the microenvironment adjacent to the apical region of the surface mucous cells to an alkaline pH.
Na+, K+, and Cl– are constituents of the protective mucosal barrier. Patients with chronic vomiting or undergoing continuous aspiration of gastric juice require intravenous replacement of NaCl, dextrose, and K+ to prevent hypokalemic metabolic acidosis.
Ménétrier's disease is a condition associated with transforming growth factor-alpha (TGF-alpha) - induced hyperplasia of surface mucous cells in the gastric mucosa.
Clinical manifestations of the disease include nausea, vomiting, epigastric pain, gastrointestinal bleeding, diarrhea, and hypoalbuminemia. The diagnosis of Ménétrier's disease is established by endoscopy (presence of large gastric folds) and biopsy, showing significant gastric pit hyperplasia with glandular atrophy and reduction in the numbers of parietal cells. Treatment includes medications to relieve nausea and gastric pain, as well as cetuximab, a monoclonal antibody that blocks TGF-alpha receptor signaling.
Chief Cells
Chief cells predominate in the lower third of the gastric gland. Chief cells are not present in cardiac glands and are seldom found in the pyloric antrum. Chief cells have a structural similarity to the zymogenic cells of the exocrine pancreas: the basal region of the cytoplasm contains an extensive rough endoplasmic reticulum. Pepsinogen-containing secretory granules (zymogen granules) are observed in the apical region of the cell.
Pepsinogen, a proenzyme stored in the zymogen granules, is released into the lumen of the gland and converted in the acid environment of the stomach to pepsin, a proteolytic enzyme capable of digesting most proteins. Exocytosis of pepsinogen is rapid and stimulated by feeding (after fasting).
Parietal cells predominate near the neck and in the upper segment of the gastric gland and are linked to chief cells by junctional complexes.
Parietal cells produce the hydrochloric acid of the gastric juice and intrinsic factor, a glycoprotein that binds to vitamin B12.
Vitamin B12 binds in the stomach to the transporting binding protein intrinsic factor. In the small intestine, the vitamin B12-intrinsic factor complex binds to intrinsic factor receptor on the surface of enterocytes in the ileum and is transported to the liver through the portal circulation.
Autoimmune gastritis is caused by autoantibodies to H+,K+-dependent ATPase, a parietal cell antigen, and intrinsic factor. Destruction of parietal cells causes a reduction in hydrochloric acid in the gastric juice (achlorhydria) and a lack of synthesis of intrinsic factor.
The resulting vitamin B12 deficiency disrupts the formation of red blood cells in the bone marrow, leading to a condition known as pernicious anemia, identified by examination of peripheral blood as megaloblastic anemia characterized by macrocytic red blood cells and hypersegmented large neutrophils.
Parietal cells have three distinctive features:
1. Abundant mitochondria, which occupy about 40% of the cell volume and provide the adenosine triphosphate (ATP) required to pump H+ ions into the lumen of the secretory canaliculus.
2. A secretory or intracellular canaliculus, formed by an invagination of the apical cell surface and continuous with the lumen of the gastric gland, which is lined by numerous microvilli.
3. An H+,K+-dependent ATPase-rich tubulovesicular system, which is distributed along the secretory canaliculus during the resting state of the parietal cell.
After stimulation, the tubulovesicular system fuses with the membrane of the secretory canaliculus, and numerous microvilli project into the canalicular space. Membrane fusion increases the amount of H+,K+-ATPase and expands the secretory canaliculus. H+,K+-ATPase represents about 80% of the protein content of the plasma membrane of the microvilli.
Secretion of Hydrochloric Acid
Parietal cells produce an acidic secretion (pH 0.9 to 2.0) rich in hydrochloric acid, with a concentration of H+ ions one million times greater than that of blood. The release of H+ ions and Cl– by the parietal cell involves the membrane fusion of the tubulovesicular system with the secretory canaliculus.
The parasympathetic (vagus nerve) mediator acetylcholine (bound to a muscarinic (M3) receptor) and the peptide gastrin, produced by enteroendocrine cells of the pyloric antrum, stimulate parietal cells to secrete HCl.
Acetylcholine also stimulates the release of gastrin. Histamine potentiates the effects of acetylcholine and gastrin on parietal cell secretion after binding to the histamine H2 receptor. Histamine is produced by enterochromaffin-like (ECL) cells within the lamina propria surrounding the gastric glands. Cimetidine is an H2 receptor antagonist that inhibits histamine-dependent acid secretion.
H+,K+-dependent ATPase facilitates the exchange of H+ and K+. Cl– and Na+ (derived from the dissociation of NaCl) are actively transported into the lumen of the secretory canaliculus, leading to the production of HCl. K+ and Na+ are recycled back into the cell by separate pumps once H+ has taken their place.
Omeprazole, with binding affinity to H+,K+-dependent ATPase, inactivates acid secretion and is an effective agent in the treatment of peptic ulcer.
Water enters the cell by osmosis, because of the secretion of ions into the canaliculus, and dissociates into H+ and hydroxyl ions (HO–). Carbon dioxide, entering the cell from the blood or formed during metabolism of the cell, combines with HO– to form carbonic acid under the influence of carbonic anhydrase. Carbonic acid dissociates into bicarbonate ions (HCO3–) and hydrogen ions. HCO3– diffuses out of the cell into the blood and accounts for the increase in blood plasma pH during digestion.
Pathology: Helicobacter Pylori Infection
The gastric juice is a combination of two separate secretions:
1. An alkaline mucosal gel protective secretion, produced by surface mucous cells and mucous neck cells.
2. HCl and pepsin, two parietal cell - chief cell - derived potentially aggressive secretions. The protective secretion is constitutive; it is always present. The aggressive secretion is facultative because hydrochloric acid and pepsin levels increase above basal levels after food intake.
The viscous, highly glycosylated gastric mucus blanket, produced by surface mucous cells and mucous neck cells, maintains a neutral pH at the epithelial cell surfaces of the stomach. In addition, the mitochondrial-rich surface mucous cells produce HCO3– ions diffusing into the surface mucus gel.
HCO3– ions, produced by parietal cells, enter the fenestrated capillaries of the lamina propria. Some of the HCO3– ions diffuse into the mucus blanket and neutralize the low pH created by the HCl content of the gastric lumen at the vicinity of the surface mucous cells.
However, the mucus blanket lining the gastric epithelium, in particular in the pyloric antrum, is the site where the flagellated bacterium Helicobacter pylori resides in spite of the hostile environment.
H. pylori survives and replicates in the gastric lumen. Its presence has been associated with acid peptic ulcers and adenocarcinoma of the stomach.
Three phases define the pathogenesis of H. pylori:
1. An active phase, in which motile bacteria increase the gastric pH by producing ammonia through the action of urease.
2. A stationary phase, consisting in the bacterial attachment to fucose-containing receptors on the surface of mucous surface cells of the pyloric region. H. pylori attachment results in the production of cytotoxic proteases that ensure the bacteria a supply of nutrients from surface mucous cells and also attract leukocytes. Both ammonia production and cytotoxic proteases correlate with the development of peptic ulcers of the pyloric mucosa.
3. During the colonization phase, H. pylori detach from the fucose-containing receptors of the surface mucus epithelium, increase in number by replication within the mucus blanket, and remain attached to glycoproteins containing sialic acid. Despite the rapid turnover of the gastric mucus-secreting cells, H. pylori avoids being flushed away with dead epithelial cells by producing urease and displaying high motility.
About 20% of the population is infected with H. pylori by age 20 years. The incidence of the infection increases to about 60% by age 60. Most infected individuals do not have clinical symptoms. Intense, sudden, persistent stomach pain (relieved by eating and antacid medications), hematemesis (blood vomit), or melena (tarlike black stool) are clinical symptoms in some patients. Increasing evidence for the infectious origin of acid peptic disease and chronic gastritis led to the implementation of antibiotic therapy for all ulcer patients shown to be infected with H. pylori.
Blood tests to detect antibodies to H. pylori and urea breath tests are effective diagnostic methods. Treatment usually consists in a combination of antibiotics, suppressors of H+,K+-dependent ATPase, and stomach protectors.
More recently, attention has been directed to adhesins and fucose-containing receptors as potential targets for drug action. The objective is to prevent binding of pathogenic bacteria without interfering with the endogenous bacterial flora by the use of antibiotics.
Gastroenteroendocrine Cells
The function of the alimentary tube is regulated by peptide hormones, produced by gastroenteroendocrine cells, and neuroendocrine mediators, produced by neurons.
Peptide hormones are synthesized by gastroenteroendocrine cells dispersed throughout the mucosa from the stomach through the colon. The population of gastroenteroendocrine cells is so large that the gastrointestinal segment is regarded as the largest endocrine organ in the body.
Gastroenteroendocrine cells are members of the APUD system, so called because of the amine precursor uptake and decarboxylation property of amino acids.
Because not all the cells accumulate amine precursors, the designation APUD has been replaced by DNES (for diffuse neuroendocrine system).
Neuroendocrine mediators are released from nerve terminals. Acetylcholine is released at the terminals of postganglionic cholinergic nerves. Gastrin-releasing peptide is released by postsynaptic neurons activated by stimulation of the vagus nerve.
Peptide hormones produced by gastrointestinal endocrine cells have the following general functions:
1. Regulation of water, electrolyte metabolism, and enzyme secretion.
2. Regulation of gastrointestinal motility and mucosal growth.
3. Stimulation of the release of other peptide hormones.
Six major gastrointestinal peptide hormones are considered: secretin, gastrin, cholecystokinin (CCK), glucose-dependent insulinotropic peptide, motilin, and ghrelin.
Secretin was the first peptide hormone to be discovered (in 1902). Secretin is released by cells in the duodenal glands of Lieberkühn when the gastric contents enter the duodenum. Secretin stimulates pancreatic and duodenal (Brunner's glands) bicarbonate and fluid release to control the gastric acid secretion (antacid effect) and regulate the pH of the duodenal contents. Secretin, together with CCK, stimulates the growth of the exocrine pancreas. In addition, secretin (and acetylcholine) stimulates chief cells to secrete pepsinogen, and inhibits gastrin release to reduce HCl secretion in the stomach.
Gastrin is produced by G cells located in the pyloric antrum. Three forms of gastrin have been described: little gastrin, or G17 (which contains 17 amino acids), big gastrin, or G34 (which contains 34 amino acids), and minigastrin, or G14 (which consists of 14 amino acids). G cells produce primarily G17. The duodenal mucosa in humans contains G cells producing mainly G34. The neuroendocrine mediator gastrin-releasing peptide regulates the release of gastrin. Somatostatin, produced by adjacent D cells, inhibits the release of gastrin.
The main function of gastrin is to stimulate the production of HCl by parietal cells. Low gastric pH inhibits further gastrin secretion.
Gastrin can also activate CCK to stimulate gallbladder contraction. Gastrin has a trophic effect on the mucosa of the small and large intestine and the fundic region of the stomach.
Gastrin stimulates the growth of ECL cells of the stomach. Continued hypersecretion of gastrin results in hyperplasia of ECL cells. ECL cells produce histamine by decarboxylation of histidine. Histamine binds to the histamine H2 receptor on parietal cells to potentiate the effect of gastrin and acetylcholine on HCl secretion. Histamine H2 receptor blocking drugs (such as cimetidine [Tagamet] and ranitidine [Zantac]) are effective inhibitors of acid secretion.
CCK is produced in the duodenum. CCK stimulates gallbladder contraction and relaxation of the sphincter of Oddi when protein- and fat-rich chyme enters the duodenum.
Glucose-dependent insulinotropic peptide (GIP), formerly called gastric-inhibitory peptide, is produced in the duodenum. GIP stimulates insulin release (insulinotropic effect) when glucose is detected in the small intestine.
Motilin is released cyclically (every 90 minutes) during fasting from the upper small intestine and stimulates gastrointestinal motility. A neural control mechanism regulates the release of motilin.
Ghrelin is produced in the stomach (fundus). Ghrelin, binds to its receptor present in growth hormone-secreting cells of the anterior hypophysis, and stimulates the secretion of growth hormone. Ghrelin plasma levels increase during fasting triggering hunger by acting on hypothalamic feeding centers.
Plasma levels of ghrelin are high in patients with Prader-Willi syndrome (caused by abnormal gene imprinting). Severe hypotonia and feeding difficulties in early infancy, followed by obesity and uncontrollable appetite, hypogonadism, and infertility are characteristics of Prader-Willi syndrome.
Clinical Significance: Zollinger-Ellison Syndrome
Patients with gastrin-secreting tumors (gastrinomas, or Zollinger-Ellison syndrome) display parietal cell hyperplasia, mucosal hypertrophy of the fundic region of the stomach, and high acid secretion independent of feeding. The secretion of gastrin is not regulated by the low gastric pH feedback mechanism.
Gastrinoma is a rare tumor of the pancreas and duodenum that causes ectopic hypersecretion of gastrin resulting in the hypersecretion of HCl by parietal cells, leading to severe peptic ulcer disease. Gastrinoma is more common in men than in women, and the age at onset is generally between 40 and 55 years of age.
The complications of gastrinomas are fulminant stomach ulceration, diarrhea (due to an inhibitory effect of water and sodium reabsorption by the small intestine due to excessive gastrin), steatorrhea (caused by inactivation of pancreatic lipase determined by the low pH), and hypokalemia.
Pyloric Glands
Pyloric glands differ from the cardiac and gastric glands in the following layers:
1. The gastric pits, or foveolae, are deeper and extend halfway through the depth of the mucosa.
2. Pyloric glands have a larger lumen and are highly branched.
The predominant epithelial cell type of the pyloric gland is a mucus-secreting cell that resembles the mucous neck cells of the gastric glands. Most of the cell contains large and pale secretory mucus and secretory granules containing lysozyme, a bacterial lytic enzyme. Occasionally, parietal cells can be found in the pyloric glands.
Enteroendocrine cells, gastrin-secreting G cells in particular, are abundant in the antrum pyloric region. Lymphoid nodules can be seen in the lamina propria.
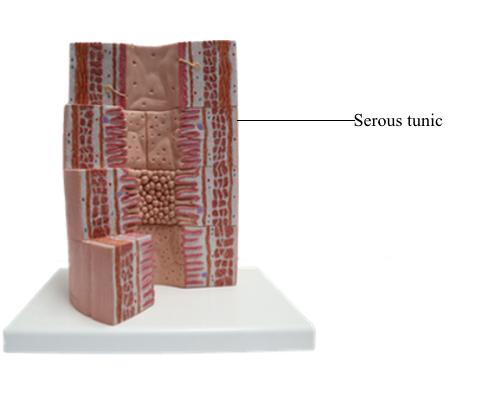
Mucosa, Submucosa, Muscularis, and Serosa of the Stomach
The mucosa consists of loose connective tissue, called the lamina propria, surrounding cardiac, gastric, and pyloric glands.
Reticular and collagen fibers predominate in the lamina propria, and elastic fibers are rare. The cell components of the lamina propria include fibroblasts, lymphocytes, mast cells, eosinophils, and a few plasma cells. The muscularis mucosae can project thin strands of muscle cells into the mucosa to facilitate the release of secretions from the gastric glands.
The submucosa consists of dense irregular connective tissue in which collagenous and elastic fibers are abundant. A large number of arterioles, venous plexuses, and lymphatics are present in the submucosa. Also present are the cell bodies and nerve fibers of the submucosal plexus of Meissner.
The muscularis (or muscularis externa) of the stomach consists of three poorly defined layers of smooth muscle oriented in circular, oblique, and longitudinal directions. At the level of the distal pyloric antrum, the circular muscle layer thickens to form the annular pyloric sphincter.
Contraction of the muscularis is under control of the autonomic nerve plexuses located between the muscle layers (myenteric plexus of Auerbach).
Based on motility functions, the stomach can be divided into two major regions:
1. The orad (Latin os [plural ora], mouth; ad, to; toward the mouth) portion, consisting of the fundus and part of the body.
2. The caudad (Latin cauda, tail; ad, to; toward the tail) portion, comprising the distal body and the antrum.
During swallowing, the orad region of the stomach and the LES relax to accommodate the ingested material. The tonus of the muscularis adjusts to the volume of the organ without increasing the pressure in the lumen.
Contraction of the caudad portion of the stomach mixes and propels the gastric contents toward the gastroduodenal junction. Most solid contents are propelled back (retropulsion) into the main body of the stomach because of the closure of the distal antrum. Liquids empty more rapidly. Retropulsion determines both mixing and mechanical dissociation of solid particles. When the gastric juice empties into the duodenum, peristaltic waves from the orad to the caudad portion of the stomach propel the contents in coordination with the relaxation of the pyloric sphincter.
The serosa consists of loose connective tissue and blood vessels of the subserosal plexus.