Main Model
BRAIN : Medial view of right side of brain
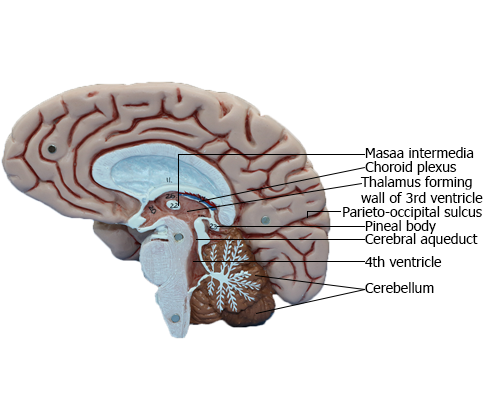
Thalamus
The gray matter of the diencephalon develops from a continuation of the brainstem alar plates; there is no homologue of the
basal plate in the diencephalon. This alar plate is recognizable at 4 weeks of gestation; by 6 weeks of gestation, it has differentiated into three main areas of the diencephalon: the epithalamus,
thalamus (dorsal thalamus), and hypothalamus.
These structures are visible as swellings in the wall of the third
ventricle, where they are separated from each other by the epithalamic and hypothalamic sulci. As development progresses,
the epithalamic area remains small, whereas the hypothalamus
and especially the thalamus enlarge. In about 80% of individuals,
the two thalami fuse across the third ventricle to form the interthalamic adhesion (massa intermedia).
The basic concepts of the development of the thalamus are the
same as for other CNS regions. Radial glia extend from the third
ventricle to the pial surface, and developing neurons migrate along this guide. The development of the thalamus occurs in an "outside-first" sequence. That is, the first neurons to undergo their final cell division migrate to the outermost portion of the thalamus, where they mature. This means that the
most lateral of the thalamic nuclei, such as the geniculate nuclei
and the lateral and ventral nuclei, are generated first. The most
medial thalamic nuclei, such as the dorsomedial nucleus, are the
last to develop.
An important process in the development of the thalamic relay
nuclei is the establishment of orderly maps of the sensory world.
For example, a retinotopic (vision) map is formed in the lateral
geniculate nucleus. As retinal ganglion cells send axons to the
lateral geniculate nucleus, the arrangement of axonal contacts on
cells in this visual relay center must accurately reflect the positions of ganglion cells in the retina. In this way, the map of visual
space on the retina is maintained in the lateral geniculate nucleus
and ultimately in the visual cortex. Similar maps are formed in
the medial geniculate nucleus (tonotopic mapping) and ventral
posterolateral nucleus (somatotopic mapping).
---------------------------------------------------------------------------------------------------------------------------------------------------------------
The ventricular spaces of the brain are the adult elaborations of
the neural canal of early developmental stages. These spaces, the
choroid plexuses in them, and the cerebrospinal fluid (CSF) produced by the choroid plexus are essential elements in the normal
function of the brain.
OVERVIEW
By about the third week of development, the nervous system
consists of a tube closed at both ends and somewhat hook shaped
rostrally (Fig. 6-1). The cavity of this tube, the neural canal,
eventually gives rise to the ventricles of the adult brain and the
central canal of the spinal cord. The ventricles become elaborate
as the various parts of the brain differentiate, whereas the central
canal becomes progressively smaller as the spinal cord differentiates into its adult pattern.
The choroid plexus, which secretes the CSF that fills the ventricles and the subarachnoid space, arises from tufts of cells that
appear in the wall of each ventricle during the first trimester.
These cells are specialized for a secretory function. The production of CSF is an active process that requires an expenditure of
energy by the choroidal cells.
Any condition that causes CSF to accumulate, such as overproduction or an obstruction of its movement through the ventricle
system, produces serious neurologic deficits. Perhaps the most
widely recognized example is hydrocephalus as seen in a fetus or in a newborn. This condition is usually caused by an obstruction
of CSF flow with resultant enlargement of the ventricular spaces
upstream to the blockage. The bones of the developing skull move
apart, and the head may enlarge significantly. In most of these cases,
some type of surgical diversion of CSF flow (a shunting procedure)
is necessary.
DEVELOPMENT
The anterior (rostral) and posterior (caudal) neuropores close at
about 24 and 26 days, respectively. At this point, the neural tube
is lined by the differentiating neuroepithelial cells of the ventricular zone, which are undergoing waves of cell division. Some
of these precursor cells give rise to the ependymal cells that line
the developing (and mature) ventricular system and the central
canal.
The brain is initially composed of three primary brain vesicles—rhombencephalon, mesencephalon, and prosencephalon—each containing a portion of the cavity of the neural tube
(Fig. 6-1A, D). The appearance of the pontine flexure in the
rhombencephalon and a progressively deepening groove that
separates the diencephalon from the telencephalon (the diencephalic-telencephalic sulcus, shortened here to telencephalic
flexure) divide these three vesicles into the five brain vesicles
(myelencephalon, metencephalon, mesencephalon, diencephalon, telencephalon) characteristic of the adult brain (Fig. 6-1B,
E). With subsequent development, the telencephalon enlarges
significantly (Fig. 6-1C, F). As the brain enlarges from three to
five vesicles, each part pulls along a portion of the cavity of the
primitive neural tube. These spaces in each brain vesicle form
the ventricle of that part of the brain in the adult. Consequently,
the shape of the ventricular system conforms, in general, to the
changes in configuration of the surrounding parts of the brain.
The lateral ventricles follow the enlarging cerebral hemispheres, and the third ventricle remains a single midline space
(Fig. 6-1A-C). The communications between the lateral ventricles and the third ventricle, the interventricular foramina (of
Monro), are initially large but become small, in proportion to the
enlarging brain, as development progresses (Fig. 6-1C, F). A colloid cyst is a type of glioma that, although comprising only about
1% of all intracranial tumors, has a predilection for being found
at the position of the interventricular foramen. In this location,
it may block the egress of CSF from the lateral ventricles and
result in enlarged ventricles and thinning of the corpus callosum. These patients usually present with headache, nausea, and
vomiting (features of increased intracranial pressure) and with
mental changes and gait disturbances. Proliferation of the neural
elements of the mesencephalon results in a reduction in the size
of the cavity of this vesicle to form the cerebral aqueduct of the
adult brain (Fig. 6-1C, F). This creates a constricted region in
the ventricular system and thus a point at which the flow of CSF
may be easily blocked. Occlusion of the cerebral aqueduct during development may be the result of glial scarring (gliosis) due
to infection or a consequence of developmental defects of the
forebrain, a rupture of the amnionic sac in utero, or forking of
the aqueduct. The last entity is a genetic sex-linked condition in which the aqueduct is reduced to two or more very small channels that do not properly meet. In addition, the cerebral aqueduct
may be reduced to such a small channel that CSF flow is reduced
or essentially blocked. Whatever the cause, occlusion of the cerebral aqueduct results in a lack of communication between the
third and fourth ventricles and blocks the egress of CSF from the
third ventricle. Caudally, the cerebral aqueduct flares open into
the fourth ventricle (Fig. 6-1B, C, E, F).
Foramina of the Fourth Ventricle
The ventricles and central canal of the spinal cord initially form
a closed system. However, in the second and third months of
development, three openings form in the roof of the fourth
ventricle, rendering the ventricular system continuous with the
subarachnoid space surrounding the brain and spinal cord. The
caudal part of the roof of the fourth ventricle consists of a layer
of ependymal cells internally and a delicate layer of connective
tissue externally (Fig. 6-2). The future apertures first appear in
the form of small bulges in the roof of the fourth ventricle. The
membrane forming the roof at these points becomes thinned and breaks down. The resultant openings are the medial foramen of
Magendie and the lateral foramina of Luschka (Fig. 6-2).
Formation of the Choroid Plexus
The caudal roof of the fourth ventricle is composed of ependymal cells on the luminal surface and a delicate layer of connective
tissue, the pia mater, on its external surface. These collectively
form the tela choroidea (Fig. 6-3). Developing arteries in the
immediate vicinity invaginate the roof of the ventricle to form a
narrow groove, the choroid fissure, in the tela choroidea. These
small developing arteries are branches of what will become the
posterior inferior cerebellar artery in the adult. The involuted
ependymal cells, along with vessels and a small amount of connective tissue, represent the primordial choroid plexus inside the
ventricular space. As development progresses, the choroid plexus
enlarges, forms many small elevations called villi, and begins to
secrete CSF (Fig. 6-3; see also Fig. 6-18). By about the end of
the first trimester, the choroid plexus is functional, the openings
in the fourth ventricle are patent, and there is circulation of CSF
through the ventricular system and into the subarachnoid space.
The choroid plexuses of the third and lateral ventricles develop
much in the same manner (Fig. 6-4). A choroid fissure appears in
the roof of the third ventricle and in the medial wall of the lateral
ventricle. The choroid plexus develops along these lines, bulges
into the respective space, and is continuous from lateral to third
ventricles through the interventricular foramen. The small arteries
serving the choroid plexus of the third ventricle are branches of
the medial posterior choroidal artery, and those serving the choroid
plexus of the lateral ventricles are branches of the lateral posterior
choroidal artery and the anterior choroidal artery (see Chapter 8).
In the adult, the choroid plexus is found in both lateral ventricles and in the third and fourth ventricles (Fig. 6-4). The development of this structure is essentially the same in all of these
spaces and is described here for the fourth ventricle.
VENTRICLES
Lateral Ventricles
The cavities of the telencephalon are the lateral ventricles, of
which there is one in each hemisphere (Fig. 6-4). As the development of the hemispheres creates the frontal, temporal, and occipital lobes, the lateral ventricles are pulled along and thus
acquire their definitive adult shape of a flattened C with a short
tail (Fig. 6-4). This shape is present by birth. The lateral ventricle
consists of an anterior horn, a body, and posterior and inferior
horns (Fig. 6-4). The junction of the body with the posterior and
inferior horns constitutes the atrium of the lateral ventricle. An
especially large clump of choroid plexus, the glomus (or glomus
choroideum), is found in the atrium (Fig. 6-4). In adults and
especially in elderly persons, the glomus may contain calcifications that are visible (as white spots) on radiographs or computed
tomography (CT) scans (Fig. 6-5). Shifts in the position of the
glomus, usually accompanied by alterations in the volume or
shape of the surrounding ventricle, may indicate some type of
ongoing pathologic process or space-occupying lesion.
The elaborate shape of the lateral ventricle means that different structures border on different parts of this space. The anterior horn and body of the lateral ventricle are bordered
medially by the septum pellucidum (at rostral levels) and by a
bundle of fibers called the fornix (at caudal levels) and posteriorly (superiorly) by the corpus callosum (Figs. 6-4 and 6-6).
The floor of the body of the lateral ventricle is made up of the
thalamus, and the caudate nucleus is characteristically found
in the lateral wall of the lateral ventricle throughout its extent
(Figs. 6-4 and 6-6). In the temporal lobe, the inferior horn of
the lateral ventricle contains the tail of the caudate nucleus in
its lateral wall, the hippocampal formation in its medial wall,
and a large group of cells (the amygdaloid complex) in its rostral end (Figs. 6-4 and 6-6).
The openings between the lateral and third ventricles, the
interventricular foramina, are located between the column of
the fornix and the rostral and medial ends of the thalamus. There
are two interventricular foramina, one opening from each lateral
ventricle into the single midline third ventricle (Fig. 6-4B).
Third Ventricle
The third ventricle, the cavity of the diencephalon, is a narrow,
vertically oriented midline space that communicates rostrally
with the lateral ventricles and caudally with the cerebral aqueduct (Figs. 6-4 and 6-8). The third ventricle has an elaborate profile on a sagittal view (Fig. 6-4A), but it is narrow in the coronal
and axial planes (Fig. 6-7).
The boundaries of the third ventricle are formed by a variety
of structures, the most important being the dorsal thalamus
and hypothalamus, and by structures that form small outpocketings called recesses (Figs. 6-4A and 6-8). These are the supraoptic recess (above the optic chiasm), the infundibular recess
(in the infundibulum, the stalk of the pituitary), the pineal
recess (in the stalk of the pineal), and the suprapineal recess
(above the pineal). The rostral wall of the third ventricle is
formed by a short segment of the anterior commissure and a
thin membrane, the lamina terminalis, that extends from the
anterior commissure anteriorly (ventrally) to the rostral edge
of the optic chiasm (Figs. 6-4A and 6-8). The floor of the third
ventricle is formed by the optic chiasm and infundibulum and their corresponding recesses plus a line extending caudally
along the rostral aspect of the midbrain to the cerebral aqueduct. The caudal wall is formed by the posterior commissure
and the recesses related to the pineal, whereas the roof is the
tela choroidea, from which the choroid plexus is suspended
(Figs. 6-4A and 6-8).
Cerebral Aqueduct
The cerebral aqueduct, the extension of the ventricle through
the mesencephalon, communicates rostrally with the third
ventricle and caudally with the fourth ventricle (Figs. 6-4A
and 6-8). This midline channel is about 1.5 mm in diameter
in adults and contains no choroid plexus. Its narrow diameter
makes it especially susceptible to occlusion. For example, cellular debris in the ventricular system (from infections or hemorrhage) may clog the aqueduct. Tumors in the area of the
midbrain (such as pinealoma) may compress the midbrain and occlude the aqueduct. The result is a blockage of CSF flow and
enlargement of the third and lateral ventricles at the expense
of the surrounding brain tissue. The cerebral aqueduct is surrounded on all sides by a sleeve of gray matter that contains
primarily small neurons; this is the periaqueductal gray or central gray.
Fourth Ventricle
The fourth ventricle is a roughly pyramid-shaped space that
forms the cavity of the metencephalon and myelencephalon
(Figs. 6-4 and 6-8). The apex of this ventricle extends into the
base of the cerebellum, and caudally it tapers to a narrow channel
that continues into the cervical spinal cord as the central canal.
Laterally the fourth ventricle extends over the surface of the
medulla as the lateral recesses, eventually to open into the area
of the pons-medulla-cerebellum junction, the cerebellopontine
angle, through the foramina of Luschka (Figs. 6-4 and 6-9). The irregularly shaped foramen of Magendie is located in the caudal
sloping roof of the ventricle (Figs. 6-4 and 6-10). Although the
roof of the caudal part of the fourth ventricle and the lateral
recesses is composed of tela choroidea, the rostral boundaries of
this space are formed by brain structures. These include the cerebellum (covering about the middle third of the ventricle) and the
superior cerebellar peduncles and anterior medullary velum (covering the rostral third of the ventricle). The floor of the fourth
ventricle, the rhomboid fossa (see Fig. 10-3), is formed by the
pons and medulla (Fig. 6-8). The only openings between the
ventricles of the brain and the subarachnoid space surrounding the brain are the foramina of Luschka and Magendie in the
fourth ventricle.
Choroid Plexus
The choroid plexus extends from the inferior horn of the lateral ventricle into the atrium (where the glomus choroideum
is located) along the floor of the body of the lateral ventricle,
continues through the interventricular foramen, and attaches
to the roof of the third ventricle (Figs. 6-4, 6-15, and 6-16). A
tumor of the choroid plexus at the point where it is continuous
through the interventricular foramen (Fig. 6-16) may result in an
enlargement of the lateral ventricle on that side with signs and symptoms of increased intracranial pressure (vomiting, lethargy,
headache, possible papilledema). Such lesions are candidates for
surgical removal. The choroid plexus of the fourth ventricle is
attached to the caudal roof and extends laterally into the foramen of Luschka (Figs. 6-4 and 6-9).
The choroid plexus in each ventricle is thrown into a series
of folds called villi (singular, villus). These are covered on their
ventricular (luminal) surfaces by a continuum of dome-shaped
structures, each with numerous microvilli (Fig. 6-17A, B). Each
dome represents the luminal surface of one choroid epithelial
cell, and the shallow grooves between domes are the points of
contact between adjacent choroid cells (Fig. 6-17B, C). Each
villus consists of a core of highly vascularized connective tissue
derived from the pia mater and a simple cuboidal covering (the
choroid epithelial cell layer), which is derived from ependymal cells (Figs. 6-3 and 6-17B, C). The abundant capillaries
in the connective tissue core of each villus are surrounded by
a basal lamina. The endothelial cells of these capillaries have
numerous fenestrations, which allow a free exchange of molecules between blood plasma and the extracellular fluid in the connective tissue core (Fig. 6-18). The connective tissue core
itself consists of fibroblasts and collagen fibrils. Another basal
lamina is formed at the interface between the connective tissue core and the choroid epithelial cells that form the surface
of each villus (Figs. 6-17C and 6-18). These choroid cells have
microvilli on their apical (ventricular) surface, interdigitating cell membranes on their sides, and irregular bases. Each is
attached to its neighbor by continuous tight junctions (zonulae occludentes) that seal off the subjacent extracellular space
from the ventricular space (Figs. 6-17C and 6-18). This represents the blood-CSF barrier. Choroid epithelial cells contain
a nucleus, numerous mitochondria, rough endoplasmic reticulum, and a small Golgi apparatus (Figs. 6-17C and 6-18). Thus
they are specialized to control the flow of ions and metabolites
into the CSF.
Although choroid epithelial cells are joined by tight junctions,
ependymal cells are not (Fig. 6-18). Therefore, fluid exchange
occurs freely between CSF and the extracellular fluid of the
brain parenchyma. The composition of CSF can thus sometimes
reflect disease processes occurring in brain tissue.
In humans the blood supply to the choroid plexuses is via the
choroidal arteries and the posterior cerebellar arteries. Choroid
plexus in the inferior horn, atrium of the lateral ventricle, and
body of the lateral ventricle is served by the anterior choroidal
artery (a branch of the internal carotid) and the lateral posterior
choroidal artery (a branch of P2). The medial posterior choroidal
artery (also a branch of P2) serves the choroid plexus of the third
ventricle. The choroid plexus located inside the fourth ventricle
is served by branches of the posterior inferior cerebellar artery
(Figs. 6-8 and 6-10), and the tuft that extends out of the foramen
of Luschka into the subarachnoid space (Fig. 6-9) is served by the
anterior inferior cerebellar artery.
Tumors of the Choroid Plexus
Tumors of the choroid plexus are relatively rare, comprising somewhat less than 1% of all intracranial tumors. In general, these lesions
are classified as choroid plexus papillomas (Fig. 6-19). These are
benign and occur more frequently than choroid plexus carcinomas,
which are malignant and rarely seen. Although these tumors may
be seen in patients of any age, they are more common between
birth and 10 years. They more often occur in the fourth ventricle
(50% to 60%) but may also be found in the lateral and third ventricles. These patients present with signs and symptoms of increased
intracranial pressure (headache, nausea, vomiting, lethargy), hydrocephalus (excessive production of CSF), or deficits of eye movement due to pressure on the roots of III, IV, or VI. The treatment
of choice for the more commonly seen tumor, a choroid plexus
papilloma, is surgical removal. The more rarely seen choroid plexus
carcinoma is treated first with chemotherapy, followed by surgery,
then with a combination of chemotherapy and radiation therapy.
On histologic examination, these tumors are characterized by
clusters of cuboidal or columnar cells that are strikingly similar to
normal choroid plexus epithelium (Fig. 6-19). These cell clusters are insinuated between comparatively thin areas containing small
vessels and loose connective tissue. This is one important difference between this tumor and an ependymoma, which has thick
intervening areas that are composed of glial cell processes (compare Figs. 6-14 and 6-19). Mitotic figures are infrequently seen
but when present may indicate that the tumor is malignant.