Main Model
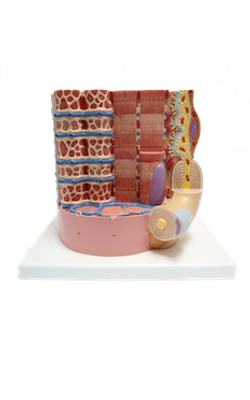
Anterior : Postsynaptic membrane
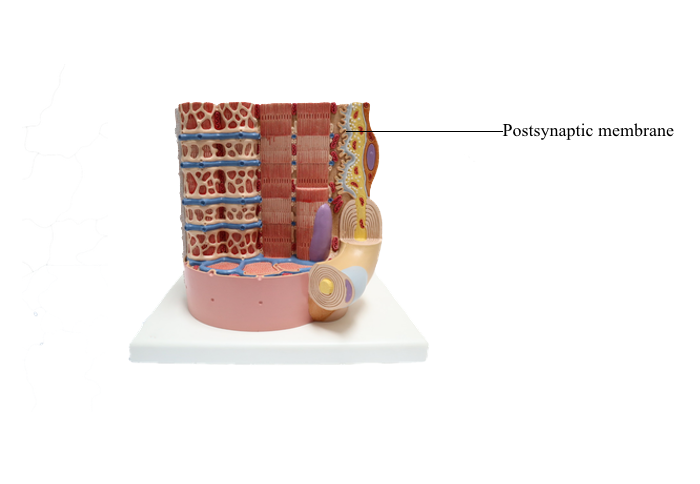
Chemical Synapses
In chemical synapses, there is a gap between the presynaptic cell membrane and the postsynaptic cell membrane, known as the synaptic cleft. Information is transmitted across the synaptic cleft via a neurotransmitter, a substance that is released from the presynaptic
terminal and binds to receptors on the postsynaptic
terminal.
The following sequence of events occurs at chemical
synapses: An action potential in the presynaptic cell
causes Ca2+ channels to open. An influx of Ca2+ into
the presynaptic terminal causes the neurotransmitter,
which is stored in synaptic vesicles, to be released by
exocytosis. The neurotransmitter diffuses across the
synaptic cleft, binds to receptors on the postsynaptic membrane, and produces a change in membrane
potential on the postsynaptic cell.
The change in membrane potential on the postsynaptic cell membrane can be either excitatory or inhibitory, depending on the nature of the neurotransmitter
released from the presynaptic nerve terminal. If the
neurotransmitter is excitatory, it causes depolarization of the postsynaptic cell; if the neurotransmitter is
inhibitory, it causes hyperpolarization of the postsynaptic cell.
In contrast to electrical synapses, neurotransmission
across chemical synapses is unidirectional (from presynaptic cell to postsynaptic cell). The synaptic delay
is the time required for the multiple steps in chemical
neurotransmission to occur.
Neuromuscular Junction - Example of
a Chemical Synapse
Motor Units
Motoneurons are the nerves that innervate muscle
fibers. A motor unit comprises a single motoneuron
and the muscle fibers it innervates. Motor units vary
considerably in size: A single motoneuron may activate
a few muscle fibers or thousands of muscle fibers.
Predictably, small motor units are involved in fine
motor activities (e.g., facial expressions), and large
motor units are involved in gross muscular activities
(e.g., quadriceps muscles used in running).
Sequence of Events at the
Neuromuscular Junction
The synapse between a motoneuron and a muscle fiber
is called the neuromuscular junction. An
action potential in the motoneuron produces an action
potential in the muscle fibers it innervates by the following sequence of events:
1. Action potentials are propagated down the motoneuron. Local currents
depolarize each adjacent region to threshold. Finally,
the presynaptic terminal is depolarized, and this depolarization causes voltage-gated Ca2+ channels
in the presynaptic membrane to open.
2. When these Ca2+ channels open, the Ca2+ permeability of the presynaptic terminal increases, and Ca2+ flows into the terminal down its electrochemical
gradient.
3. Ca2+ uptake into the terminal causes release of the
neurotransmitter acetylcholine (ACh), which has
been previously synthesized and stored in synaptic
vesicles. To release ACh, the synaptic vesicles fuse
with the plasma membrane and empty their contents into the synaptic cleft by exocytosis.
ACh is formed from acetyl coenzyme A (acetyl
CoA) and choline by the action of the enzyme choline acetyltransferase. ACh is stored
in vesicles with ATP and proteoglycan for subsequent release. On stimulation, the entire content of
a synaptic vesicle is released into the synaptic cleft.
The smallest possible amount of ACh that can be
released is the content of one synaptic vesicle (one
quantum), and for this reason, the release of ACh
is said to be quantal.
4. ACh diffuses across the synaptic cleft to the postsynaptic membrane. This specialized region of the
muscle fiber is called the motor end plate, which
contains nicotinic receptors for ACh. ACh binds to
the α subunits of the nicotinic receptor and causes
a conformational change. It is important to note that
the nicotinic receptor for ACh is an example of a
ligand-gated ion channel: It also is an Na+ and K+channel. When the conformational change occurs,
the central core of the channel opens, and the permeability of the motor end plate to both Na+ and K+ increases.
5. When these channels open, both Na+ and K+ flow
down their respective electrochemical gradients,
Na+ moving into the end plate and K+ moving
out, each ion attempting to drive the motor end
plate potential to its equilibrium potential. Indeed,
if there were no other ion channels in the motor
end plate, the end plate would depolarize to a value
about halfway between the equilibrium potentials
for Na+ and K+, or approximately 0 mV. (In this
case, zero is not a "magic number" - it simply
happens to be the value about halfway between the
two equilibrium potentials.) In practice, however,
because other ion channels that influence membrane potential are present in the end plate, the
motor end plate only depolarizes to about -50 mV,
which is the end plate potential (EPP). The EPP is
not an action potential but is simply a local depolarization of the specialized motor end plate.
The content of a single synaptic vesicle produces
the smallest possible change in membrane potential
of the motor end plate, the miniature end plate
potential (MEPP). MEPPs summate to produce the
full-fledged EPP. The spontaneous appearance of
MEPPs proves the quantal nature of ACh release at
the neuromuscular junction.
Each MEPP, which represents the content of one
synaptic vesicle, depolarizes the motor end plate by
about 0.4 mV. An EPP is a multiple of these 0.4 mV
units of depolarization. How many such quanta are
required to depolarize the motor end plate to the
EPP? Because the motor end plate must be depolarized from its resting potential of -90 mV to the
threshold potential of -50 mV, it must, therefore,
depolarize by 40 mV. Depolarization by 40 mV
requires 100 quanta (because each quantum or
vesicle depolarizes the motor end plate by 0.4 mV).
6. Depolarization of the motor end plate (the EPP)
then spreads by local currents to adjacent muscle
fibers, which are depolarized to threshold and fire
action potentials. Although the motor end plate
itself cannot fire action potentials, it depolarizes
sufficiently to initiate the process in the neighboring "regular" muscle cells. Action potentials are propagated down the muscle fiber by a continuation of
this process.
7. The EPP at the motor end plate is terminated when
ACh is degraded to choline and acetate by acetylcholinesterase (AChE) on the motor end plate.
Approximately 50% of the choline is returned to the
presynaptic terminal by Na+-choline cotransport, to
be used again in the synthesis of new ACh.
Agents That Alter Neuromuscular Function
Several agents interfere with normal activity at the neuromuscular junction, and their mechanisms of action
can be readily understood by considering the steps
involved in neuromuscular transmission.
♦ Botulinus toxin blocks the release of ACh from presynaptic terminals, causing total blockade of neuromuscular transmission, paralysis of skeletal muscle,
and, eventually, death from respiratory failure.
♦ Curare competes with ACh for the nicotinic receptors on the motor end plate, decreasing the size of
the EPP. When administered in maximal doses,
curare causes paralysis and death. D-Tubocurarine,
a form of curare, is used therapeutically to cause
relaxation of skeletal muscle during anesthesia.
A related substance, α-bungarotoxin, binds irreversibly to ACh receptors. Binding of radioactive
α-bungarotoxin has provided an experimental tool
for measuring the density of ACh receptors on the
motor end plate.
♦ AChE inhibitors (anticholinesterases) such as neostigmine prevent degradation of ACh in the synaptic
cleft, and they prolong and enhance the action of
ACh at the motor end plate. AChE inhibitors can be
used in the treatment of myasthenia gravis, a disease characterized by skeletal muscle weakness
and fatigability, in which ACh receptors are blocked
by antibodies.
♦ Hemicholinium blocks choline reuptake into presynaptic terminals, thus depleting choline stores
from the motoneuron terminal and decreasing the
synthesis of ACh.