Main Model
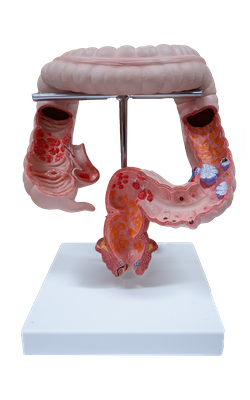
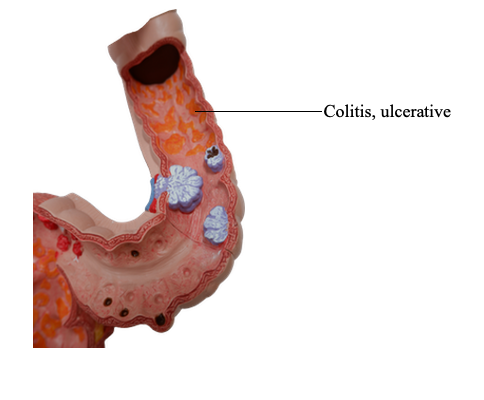
Colitis, ulcerative
Inflammatory Intestinal Disease
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a chronic condition resulting from complex interactions between intestinal microbiota and host immunity in genetically predisposed individuals resulting an inappropriate mucosal immune activation. IBD encompasses two entities, Crohn disease and ulcerative colitis. The distinction between ulcerative colitis and Crohn disease is based, in large part, on the distribution of affected sites and the morphologic expression of disease at those sites. Ulcerative colitis is limited to the colon and rectum and extends only into the mucosa and submucosa. By contrast, Crohn disease, also referred to as regional enteritis (because of frequent ileal involvement), may involve any area of the gastrointestinal tract and is frequently transmural.
Epidemiology
Both Crohn disease and ulcerative colitis frequently present during adolescence or in young adults, although some studies suggest a second, smaller peak in the incidence of both diseases after the fifth decade. In Western industrialized nations, IBD is most common among whites and, in the United States, occurs three to five times more often among eastern European (Ashkenazi) Jews. This predilection is at least partly due to genetic factors, as discussed below. The geographic distribution of IBD is highly variable, but it is most prevalent in North America, northern Europe, and Australia. The incidence of IBD worldwide is on the rise and it is becoming more common in regions in which the prevalence was historically low. The hygiene hypothesis, first applied to asthma, says that childhood and even prenatal exposure to environmental microbes resets the immune system in a way that prevents excessive reactions. Extrapolated to IBD, it suggests that a reduced frequency of enteric infections due to improved hygiene has resulted in inadequate development of regulatory processes that limit mucosal immune responses early in life. While attractive and commonly stated, firm evidence is lacking and hence the increasing incidence of IBD remains mysterious.
Pathogenesis
Although precise causes are not yet defined, most investigators believe that IBD results from the combined effects of alterations in host interactions with intestinal microbiota, intestinal epithelial dysfunction, aberrant mucosal immune responses, and altered composition of the gut microbiome. This view is supported by epidemiologic, genetic, and clinical studies as well as data from laboratory models of IBD.
• Genetics. Risk for disease is increased when there is an affected family member, and in Crohn disease, the concordance rate for monozygotic twins is approximately 50%. By contrast, concordance of monozygotic twins for ulcerative colitis is only 16%, suggesting that genetic factors are less dominant in this form of IBD.
- Molecular linkage analyses of affected families have identified NOD2 (nucleotide oligomerization binding domain 2) as a susceptibility gene in Crohn disease. NOD2 encodes a protein that binds to intracellular bacterial peptidoglycans and subsequently activates NF-κB. Some studies suggest that the disease-associated form of NOD-2 is ineffective at defending against intestinal bacteria. The result is that bacteria are able to enter through the epithelium into the wall of the intestine, where they trigger inflammatory reactions. It should, however, be recognized that disease develops in less than 10% of individuals carrying specific NOD2 polymorphisms, and these polymorphisms are uncommon in African and Asian patients with Crohn disease.
- The search for IBD-associated genes using genomewide association studies (GWAS) that assess single-nucleotide polymorphisms (SNPs) as well as high throughput sequencing and other approaches have yielded a rich harvest of over 200 genes associated with IBD. Among these, NOD2, discussed above, and two autophagy-related genes are of particular interest. They are ATG16L1 (autophagy-related 16-like-1) and IRGM (immunity-related GTPase M) genes. Both are part of the autophagosome pathway and, like NOD-2, are involved in host cell responses to intracellular bacteria, supporting the hypothesis that inadequate defense against luminal bacteria may be important in the pathogenesis of IBD. None of these genes is associated with ulcerative colitis.
• Mucosal immune responses. Although the mechanisms by which mucosal immunity contributes to the pathogenesis of ulcerative colitis and Crohn disease are still being deciphered, immunosuppressive and immunomodulatory agents remain mainstays of IBD therapy. Polarization of helper T cells to the TH1 type is well recognized in Crohn disease, and some data suggest that TH17 T cells also contribute to disease pathogenesis. Consistent with this, certain polymorphisms of the IL-23 receptor confer protection from Crohn disease and ulcerative colitis (IL-23 is involved in the development and maintenance of TH17 cells). However, agents that block IL-17 or its receptor have provided no benefit, whereas an antibody that inhibits both TH1- and TH17-inducing cytokines is effective, suggesting that the two T cell subsets may have a synergistic role in the disease. Some data suggest that mucosal production of the TH2-derived cytokine IL-13 is increased in ulcerative colitis, and, to a lesser degree, Crohn disease.
Defects in regulatory T cells, especially the IL-10-producing subset, are believed to underlie the inflammation especially in Crohn disease. Mutations in the IL-10 receptor are associated with severe, early-onset colitis. Thus, some combination of excessive immune activation by intestinal microbes and defective immune regulation likely is responsible for the chronic inflammation in both forms of IBD.
• Epithelial defects. A variety of epithelial defects have been described in Crohn disease, ulcerative colitis, or both. For example, defects in intestinal epithelial tight junction barrier function occur in patients with Crohn disease and a subset of their healthy first-degree relatives. This barrier dysfunction cosegregates with specific disease-associated NOD2 polymorphisms, and experimental models demonstrate that barrier dysfunction can activate innate and adaptive mucosal immunity and sensitize subjects to disease. Interestingly, the Paneth cell granules, which contain anti-microbial peptides that can affect composition of the luminal microbiota, are abnormal in patients with Crohn disease carrying ATG16L1 mutations, thus providing one potential mechanism in which a defective feedback loop between the epithelium and microbiota could contribute to disease pathogenesis.
• Microbiota. The quantity of microbial organisms in the gastrointestinal lumen is enormous, amounting to as many as 1012 organisms/mL of fecal material in the colon (50% of fecal mass). There is significant interindividual variation in the composition of this microbial population, which is modified by diet and disease. Microbial transfer studies are able to promote or reduce disease in animal models of IBD, and clinical trials suggest that probiotic (or beneficial) bacteria or even fecal microbial transplants from healthy individuals may benefit IBD patients.
One model that unifies the roles of intestinal microbiota, epithelial function, and mucosal immunity suggests a cycle by which transepithelial flux of luminal bacterial components activates innate and adaptive immune responses. In a genetically susceptible host, the subsequent release of TNF and other immune signals directs epithelia to increase tight junction permeability, which further increases the flux of luminal material. These events may establish a self-amplifying cycle in which a stimulus at any site may be sufficient to initiate IBD.
With this background of pathogenesis, the morphologic and clinical features of each of the two forms of IBD will be discussed next.
Crohn Disease
Morphology
Crohn disease, also known as regional enteritis, may occur in any area of the gastrointestinal tract but the most common sites involved at presentation are the terminal ileum, ileocecal valve, and cecum. Disease is limited to the small intestine alone in about 40% of cases; the small intestine and the colon both are involved in 30% of patients; and the remainder of cases are characterized by colonic involvement only. Infrequently, Crohn disease may involve the esophagus or stomach.The presence of multiple, separate, sharply delineated areas of disease, resulting in skip lesions, is characteristic of Crohn disease and may help in differentiation from ulcerative colitis. Strictures are common.
The earliest lesion, the aphthous ulcer, may progress, and multiple lesions often coalesce into elongated, serpentine ulcers oriented along the axis of the bowel. Edema and loss of normal mucosal folds are common. Sparing of interspersed mucosa results in a coarsely textured, cobblestone appearance in which diseased tissue is depressed below the level of normal mucosa. Fissures frequently develop between mucosal folds and may extend deeply to become sites of perforation or fistula tracts. The intestinal wall is thickened as a consequence of transmural edema, inflammation, submucosal fibrosis, and hypertrophy of the muscularis propria, all of which contribute to stricture formation. In cases with extensive transmural disease, mesenteric fat frequently extends around the serosal surface (creeping fat).
The microscopic features of active Crohn disease include abundant neutrophils that infiltrate and damage crypt epithelium. Clusters of neutrophils within a crypt are referred to as a crypt abscess and often are associated with crypt destruction. Ulceration is common in Crohn disease, and there may be an abrupt transition between ulcerated and normal mucosa. Repeated cycles of crypt destruction and regeneration lead to distortion of mucosal architecture; the normally straight and parallel crypts take on bizarre branching shapes and unusual orientations to one another. Epithelial metaplasia, another consequence of chronic relapsing injury, often takes the form of gastric antral-appearing glands (pseudopyloric metaplasia). Paneth cell metaplasia may occur in the left colon, where Paneth cells are normally absent. These architectural and metaplastic changes may persist, even when active inflammation has resolved. Mucosal atrophy, with loss of crypts, may follow years of disease. Noncaseating granulomas, a hallmark of Crohn disease, are found in approximately 35% of cases and may arise in areas of active disease or uninvolved regions in any layer of the intestinal wall. Granulomas also may be found in mesenteric lymph nodes. Cutaneous granulomas form nodules that are referred to (misleadingly) as metastatic Crohn disease. The absence of granulomas does not preclude a diagnosis of Crohn disease.
Clinical Features
The clinical manifestations of Crohn disease are extremely variable. In most patients, disease begins with intermittent attacks of relatively mild diarrhea, fever, and abdominal pain. Approximately 20% of patients present acutely with right lower-quadrant pain and fever, which may mimic acute appendicitis or bowel perforation. Patients with colonic involvement may present with bloody diarrhea and abdominal pain, creating a differential diagnosis with some colonic infections. Periods of disease activity typically are interrupted by asymptomatic intervals that last for weeks to many months. Disease reactivation can be associated with a variety of external triggers, including physical or emotional stress, specific dietary items, NSAID use, and cigarette smoking.
Iron-deficiency anemia may develop in individuals with colonic disease, while extensive small-bowel disease may result in serum protein loss and hypoalbuminemia, generalized nutrient malabsorption, or malabsorption of vitamin B12 and bile salts. Fibrosing strictures, particularly of the terminal ileum, are common and require surgical resection. Disease often recurs at the site of anastomosis, and as many as 40% of patients require additional resections within 10 years. Fistulas develop between loops of bowel and may also involve the urinary bladder, vagina, and abdominal or perianal skin. Perforations and peritoneal abscesses can also occur.
Extraintestinal manifestations of Crohn disease include uveitis, migratory polyarthritis, sacroiliitis, ankylosing spondylitis, erythema nodosum, and clubbing of the fingertips, any of which may develop before intestinal disease is recognized. Pericholangitis and primary sclerosing cholangitis may occur in Crohn disease but are more common in ulcerative colitis. As discussed later, the risk for development of colonic adenocarcinoma is increased in patients with long-standing colonic Crohn disease.
Ulcerative Colitis
Morphology
Ulcerative colitis always involves the rectum and extends proximally in a continuous fashion to involve part or the entire colon that can be diffusely ulcerated. Skip lesions are not seen (although focal appendiceal or cecal inflammation occasionally may be present in those with left-sided disease). Disease of the entire colon is termed pancolitis. Disease limited to the rectum or rectosigmoid may be referred to descriptively as ulcerative proctitis or ulcerative proctosigmoiditis. The small intestine is normal, although mild mucosal inflammation of the distal ileum, backwash ileitis, may be present in severe cases of pancolitis.
On gross evaluation, involved colonic mucosa may be slightly red and granular-appearing or exhibit extensive broad-based ulcers. The transition between diseased and uninvolved colon can be abrupt. Ulcers are aligned along the long axis of the colon but typically do not replicate the serpentine ulcers of Crohn disease. Isolated islands of regenerating mucosa often bulge into the lumen to create small elevations, termed pseudopolyps. Chronic disease may lead to mucosal atrophy and a flat, smooth mucosal surface lacking normal folds. Unlike in Crohn disease, mural thickening is absent, the serosal surface is normal, and strictures do not occur. However, inflammation and inflammatory mediators can damage the muscularis propria and disturb neuromuscular function leading to colonic dilation and toxic megacolon, which carries a significant risk for perforation.
Histologic features of mucosal disease in ulcerative colitis are similar to those in colonic Crohn disease and include inflammatory infiltrates, crypt abscesses, crypt distortion, and epithelial metaplasia. However, skip lesions are absent, and inflammation generally is limited to the mucosa and superficial submucosa. This distinction may not be demonstrated by endoscopic biopsies, which typically sample the mucosa and little or no submucosa. In severe cases, mucosal damage may be accompanied by ulcers that extend more deeply into the submucosa, but the muscularis propria is rarely involved. Submucosal fibrosis, mucosal atrophy, and distorted mucosal architecture remain as residua of healed disease, but the histologic pattern also may revert to near normal after prolonged remission. Granulomas are not present.
Some extraintestinal manifestations of ulcerative colitis overlap with those of Crohn disease, including migratory polyarthritis, sacroiliitis, ankylosing spondylitis, uveitis, skin lesions, pericholangitis, and primary sclerosing cholangitis.
Clinical Features
Ulcerative colitis is a relapsing disorder characterized by attacks of bloody diarrhea with expulsion of stringy, mucoid material and lower abdominal pain and cramps that are temporarily relieved by defecation. These symptoms may persist for days, weeks, or months before they subside, and occasionally the initial attack may be severe enough to constitute a medical or surgical emergency. More than half of patients have mild disease, but almost all experience at least one relapse during a 10-year period. Colectomy cures intestinal disease, but extraintestinal manifestations may persist.
The factors that trigger ulcerative colitis are not known; infectious enteritis precedes disease onset in some cases. The onset of symptoms can occur shortly after smoking cessation in some patients, and smoking may partially relieve symptoms. Unfortunately, studies of nicotine as a therapeutic agent have been disappointing.
Colitis-Associated Neoplasia
One of the most feared long-term complications of ulcerative colitis and colonic Crohn disease is the development of neoplasia. This process begins as dysplasia, which, just as in Barrett esophagus and chronic gastritis, is a step along the road to full-blown carcinoma. The risk for development of dysplasia is related to several factors:
• Duration of disease. Risk increases beginning 8 to 10 years after disease initiation.
• Extent of involvement. Patients with pancolitis are at greater risk than those with only left-sided disease.
• Inflammation. Greater frequency and severity of active inflammation (characterized by the presence of neutrophils) may increase risk. This is another example of the enabling effect of inflammation on carcinogenesis.
To facilitate early detection of neoplasia, patients typically are enrolled in surveillance programs approximately 8 years after diagnosis of IBD. An important exception to this approach is in patients with primary sclerosing cholangitis, who are at markedly greater risk for development of dysplasia and generally are enrolled for surveillance at the time of diagnosis. Surveillance requires regular and extensive mucosal biopsy, making it a costly practice. In many cases, dysplasia occurs in flat areas of mucosa that do not appear abnormal by eye. Thus, advanced endoscopic imaging techniques are being developed to try to enable the detection of early dysplastic changes.
Summary
Inflammatory Bowel Disease
• Inflammatory bowel disease (IBD) is an umbrella term for Crohn disease and ulcerative colitis.
• Crohn disease most commonly affects the terminal ileum and cecum, but any site within the gastrointestinal tract can be involved; skip lesions and noncaseating granulomas are common.
• Ulcerative colitis is limited to the colon, is continuous from the rectum, and ranges in extent from only rectal disease to pancolitis; neither skip lesions nor granulomas are present.
• Both Crohn disease and ulcerative colitis can have extraintestinal manifestations.
• IBD is thought to arise from a combination of alterations in host interactions with intestinal microbiota, intestinal epithelial dysfunction, and aberrant mucosal immune responses.
• The risk for development of colonic epithelial dysplasia and adenocarcinoma is increased in patients who have had colonic IBD for more than 8 to 10 years.