Main Model
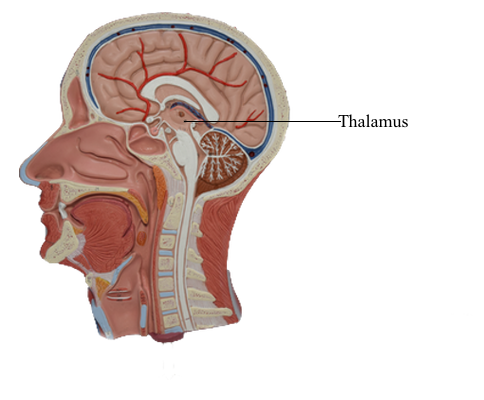
Brain : 10 Thalamus
Although it is considered by some investigators to be part of
the brainstem, the diencephalon is treated here as a portion of
the forebrain. The diencephalon includes the dorsal thalamus, hypothalamus, ventral thalamus, and epithalamus, and it is situated between the telencephalon and the brainstem. In general,
the diencephalon is the main processing center for information destined to reach the cerebral cortex from all ascending sensory
pathways (except those related to olfaction). The right and left
halves of the diencephalon, for the most part, contain symmetrically distributed cell groups separated by the space of the third
ventricle.
Overview
The dorsal thalamus, or thalamus as it is commonly called, is
the largest of the four principal subdivisions of the diencephalon and consists of pools of neurons that collectively project to nearly all areas of the cerebral cortex. Some of the thalamic
nuclei receive somatosensory, visual, or auditory input and
transmit this information to the appropriate area of the cerebral cortex. Other thalamic nuclei receive input from subcortical motor areas and project to those parts of the overlying
cortex that influence the successful execution of a motor act.
A few thalamic nuclei receive a more diffuse input and accordingly relate in a more diffuse way to widespread areas of the
cortex.
The hypothalamus is also composed of multiple nuclear subdivisions and is connected primarily to portions of the forebrain,
brainstem, and spinal cord. This part of the diencephalon is involved in the control of visceromotor (autonomic) functions.
In this respect, the hypothalamus regulates functions that are "automatically" adjusted (such as blood pressure and body temperature) without our being aware of the change. In contrast,
conscious sensation and some aspects of motor control are mediated by the dorsal thalamus.
The ventral thalamus and epithalamus are the smallest subdivisions of the diencephalon. The ventral thalamus includes
the subthalamic nucleus, which is linked to the basal nuclei of
the forebrain and functions in the motor sphere; lesions in the
subthalamus give rise to characteristic involuntary movement
disorders. The epithalamus is functionally related to the limbic
system.
Basic Organization
The junction between the diencephalon and midbrain lies along a
line extending from the posterior commissure to the caudal edge
of the mammillary body on the medial aspect of the hemisphere. On the surface of the hemisphere, this interface is
represented by a line starting at the caudal aspect of the mammillary body, extending anterolaterally over the edge of the crus
cerebri, and following the caudal edge of the optic tract. The boundary between the diencephalon and surrounding telencephalon is less distinct and is represented laterally by the internal capsule and rostrally by the interventricular foramen,
lamina terminalis, and optic chiasm.
The cavity of the diencephalon, the third ventricle, is a narrow, vertically oriented midline space located between the paired
dorsal thalami and hypothalami of the two sides. In addition to its connections with the lateral ventricles
and the cerebral aqueduct, the third ventricle has small evaginations or recesses associated with the optic chiasm (supraoptic
recess), the infundibulum (infundibular recess), and the pineal
gland (pineal and suprapineal recesses).
All four diencephalic subdivisions can be approximated in a
midsagittal section of the forebrain. The
dorsal thalamus is located superior to the hypothalamic sulcus
and extends from the interventricular foramen caudally to the
level of the splenium of the corpus callosum. The hypothalamus
lies inferior to the hypothalamic sulcus and is bordered rostrally
by the lamina terminalis and caudally by a line that extends from
the posterior aspect of the mammillary body superiorly to intersect with the hypothalamic sulcus. The only diencephalic structures visible on the inferior surface of the hemisphere are those
related to the hypothalamus, including the optic chiasm, infundibulum, medial and lateral eminences, and mammillary bodies. The ventral thalamus (subthalamus) does not border on the ventricle; rather, it occupies a position caudal to the
hypothalamus, rostral to the diencephalon-midbrain junction, and lateral to the midline. Epithalamic
structures are located posteriorly and caudally, in close apposition to the posterior commissure, and include the pineal gland,
the habenular nuclei, and the main afferent bundle of these
nuclei, the stria medullaris thalami.
Dorsal Thalamus (Thalamus)
The dorsal thalamus (or thalamus) is a massive collection of neuronal cell groups that participate in
a widely diverse array of functions involving motor, sensory, and
limbic systems. It receives a variety of ascending inputs and projects, via thalamocortical fibers to various cortical areas or gyri,
and receives reciprocal connections, via corticothalamic fibers,
from those cortical targets to which it sends projections. As a
result, the thalamus is often regarded as the functional "gateway" to the cerebral cortex.
The thalamus is covered on its lateral aspect by a layer of myelinated axons, the external medullary lamina, which includes fibers
that enter or leave the subcortical white matter.
Within the external medullary lamina are clusters of neurons that
form the thalamic reticular nucleus. The medial surface of the
thalamus borders the third ventricle, and the external medullary lamina and thalamic reticular nucleus blend with the thalamic fasciculus and zona incerta, respectively, to form an interface
between dorsal and ventral thalami.
An internal medullary lamina, also consisting of myelinated
fibers, extends into the substance of the thalamus, where it forms
partitions or boundaries that divide the thalamus into its principal
cell groups: the anterior, medial, lateral,
and intralaminar nuclear groups. The last cell group is located in
the portion of the internal medullary lamina that separates the
lateral and medial nuclear groups. In addition, there are midline
thalamic nuclei located just superior to the hypothalamic sulcus.
Finally, attached to the caudolateral portion of the thalamus
are the medial and lateral geniculate bodies (and their correspondingly named subjacent nuclei). Although considered here as components of the lateral
nuclear group, the geniculate nuclei are sometimes considered as
a separate part of the thalamus, the metathalamus.
Anterior Thalamic Nuclei
The anterior nucleus forms a prominent wedge on the rostral
aspect of the dorsal thalamus just caudolateral to the interventricular foramen; this wedge is the anterior thalamic tubercle. Internal to the anterior tubercle is a large principal nucleus and
two smaller nuclei that collectively form the anterior nucleus of
the thalamus. Rostrally,
the internal medullary lamina divides to partially encapsulate
the anterior nucleus. The cells of this nucleus receive dense limbic-related projections from (1) the mammillary nuclei via the
mammillothalamic tract and (2) the medial temporal lobe (hippocampus) via the fornix. The output of this nucleus is primarily
directed to the cingulate gyrus through the anterior limb of the
internal capsule. The anterior nucleus
is an important synaptic station in the Papez circuit, which is
related to emotion and memory acquisition.
Medial Thalamic Nuclei
This region of the dorsal thalamus comprises the dorsomedial
nucleus. This expansive group of neuronal cell bodies is composed of large parvicellular (located caudally) and magnocellular (located rostrally) parts and a small
paralaminar part adjacent to the internal medullary lamina. The two larger portions are linked to parts
of the frontal and temporal lobes and to the amygdaloid complex. Cells of the paralaminar subdivision receive input
from the frontal lobe and substantia nigra and may play a role in
the control of eye movement.
Lateral Thalamic Nuclei
This large collection of thalamic neurons is grouped into dorsal
and ventral tiers. The relatively small group of dorsal tier nuclei
includes the lateral dorsal and lateral posterior nuclei along with
the much larger pulvinar nucleus (pulvinar). The connections of the lateral dorsal
and lateral posterior nuclei are formed with the cingulate gyrus
and parietal lobe, respectively. The large pulvinar
nucleus consists of anterior, medial, lateral, and inferior subdivisions. The inferior division receives input from the superior
colliculus and projects to the visual association cortex. Other
portions of the pulvinar project to areas of the temporal, parietal,
and frontal lobes that are especially concerned with visual function and eye movements.
The large ventral tier of the lateral group consists of three
separate nuclei. The ventral
anterior nucleus (VA) and the slightly more caudal ventral lateral nucleus (VL) are important motor-related nuclei; the ventral posterior nucleus, consisting of ventral posterolateral (VPL)
and ventral posteromedial (VPM) nuclei, convey somatosensory
information to the cerebral cortex.
The VA is composed of a large parvocellular portion and a
small magnocellular part. The former receives input from the
medial segment of the globus pallidus, and the latter receives
afferents from the reticular portion of substantia nigra. The efferent projections from the VA are diffuse and appear to
include selected parts of the frontal lobe.
The VL is also composed of three
subdivisions: a pars oralis, a pars medialis, and a pars caudalis.
The largest of these, the pars oralis, receives a dense projection
from the internal segment of the ipsilateral globus pallidus; some
of these afferents enter the caudal subdivision. In contrast, the
pars caudalis subdivision of the VL receives its main input from
the contralateral cerebellar nuclei. Consequently, pallidal and
cerebellar projections are largely segregated within this nucleus.
The output of the VL reflects its segregated input in that the oral
and caudal parts project to largely separate areas of the frontal
lobe.
The larger and more laterally located VPL nucleus and the
comparatively smaller and more medially located VPM nucleus
both receive somatosensory input from the contralateral side of the body. The medial lemniscus and
spinothalamic fibers terminate in a somatotopic manner (cervical fibers medial, sacral fibers lateral) within the VPL, whereas
trigeminothalamic fibers from the spinal trigeminal nucleus and the principal trigeminal sensory nucleus terminate in the VPM.
Both the VPL and VPM project to the somatosensory cortex of
the parietal lobe.
A small group of cells called the ventral posterior inferior
nucleus is situated ventrally between the VPL and VPM. These
cells process vestibular input and project to lateral areas of the postcentral gyrus that are located in the depths of the central
sulcus. Similarly, a small group of cells forming the rostral (oral)
portion of the VPL receives cerebellar input and projects to the
precentral gyrus of the frontal lobe; this nucleus probably represents a few cells that have been displaced from the slightly more
rostrally located VL. This cell group is also called the ventral
intermediate nucleus because of its location between the VL
and VPL.
The medial (MGB) and lateral (LGB) geniculate nuclei are
considered parts of the lateral thalamic nuclear group. The MGB receives ascending auditory input via the brachium of the inferior colliculus and projects
to the primary auditory cortex in the temporal lobe. The LGB receives visual input from the retina via the optic tract and in
turn projects to the primary visual cortex on the medial surface
of the occipital lobe.
Located in the posterior thalamus at about the level of the
pulvinar and geniculate nuclei is a cluster of cell groups collectively called the posterior nuclear complex. This complex consists of the suprageniculate nucleus, the nucleus limitans, and the
posterior nucleus. These nuclei are positioned superior to the
medial geniculate and medial to the rostral pulvinar. The posterior nuclear complex receives and sends to the cortex nociceptive cutaneous input that is transmitted over somatosensory
pathways.
Intralaminar Nuclei
Embedded within the internal medullary lamina are the discontinuous groups of neurons that form the intralaminar nuclei.
These cells are characterized by their projections to the neostriatum and to other thalamic nuclei, along with diffuse projections to the cerebral cortex. Two of the most prominent
cell groups are the centromedian and parafascicular nuclei. The centromedian nucleus projects to the neostriatum and to motor areas of the cerebral cortex, whereas the parafascicular nucleus projects to rostral and lateral areas of the frontal lobe. Other intralaminar nuclei receive
input from ascending pain pathways and project to the somatosensory and parietal cortex.
Midline Nuclei
The midline nuclei are the least understood components of the
thalamus. The largest is the paratenial nucleus, which is located
just ventral to the rostral portion of the stria medullaris thalami;
other cells are associated with the interthalamic adhesion (massa
intermedia). Although inputs are poorly defined, efferent fibers
reach the amygdaloid complex and the anterior cingulate cortex,
suggesting a role in the limbic system.
Thalamic Reticular Nucleus
The cells of this nucleus are situated within the external medullary lamina and between this lamina and the internal capsule. Axons of these cells project medially
into the nuclei of the dorsal thalamus or to other parts of the
reticular nucleus, but not into the cerebral cortex. Afferents
are received from the cortex and from nuclei of the dorsal
thalamus via collaterals of thalamocortical and corticothalamic
axons. It appears that thalamic reticular neurons modulate, or
gate, the responses of thalamic neurons to incoming cerebral
cortical input.
Summary of Thalamic Organization
Each thalamic nucleus (with a few exceptions) gives rise to efferent projections (thalamocortical fibers) that target some portion
of the cerebral cortex. That region of cortex then typically provides a reciprocal projection (corticothalamic fibers) that returns
to the original thalamic nucleus.
Some thalamic nuclei are primarily associated with a particular function and in turn with a specific gyrus (and functional area)
of the cerebral cortex. The more important of these relationships
are as follows: VL/motor/precentral gyrus and anterior paracentral gyrus; VPL/sensory for the body/postcentral gyrus and posterior paracentral gyrus; VPM/sensory for the face/postcentral
gyrus; MGB/auditory/transverse temporal gyrus; LGB/vision/
cortex on the calcarine sulcus. The anterior nucleus projects primarily to the cingulate gyrus and functions in the broad area of
behavior.
The nuclei of the thalamus have been classified according to
their connections as either relay nuclei or association nuclei. A
relay nucleus is one that receives input predominantly from a single source, such as a sensory pathway or a cerebellar nucleus,
or from the basal nuclei. The incoming neural information is processed and then sent to a localized region of sensory, motor, or
limbic cortex. Relay nuclei include MGB, LGB, VPL, VPM, VL,
VA, and the anterior thalamic nuclei. These nuclei do not merely
relay neural signals; in fact, considerable neural processing also
takes place in these nuclei. However, their position in a modality-specific pathway linking one particular source to one particular
destination makes the word "relay" a useful designation. In contrast, an association nucleus receives input from a number of different structures or cortical regions and usually sends its output
to more than one of the association areas of the cerebral cortex
(i.e., areas that are neither sensory nor motor cortex). Association nuclei include dorsomedial, lateral dorsal, lateral posterior, and the nuclei of the pulvinar complex.
A thalamic nucleus can also be designated specific or nonspecific on the basis of thalamocortical signals generated in response
to electrical stimulation delivered to a localized site in that thalamic nucleus. Focal electrical stimulation of a specific nucleus
produces a rapidly conducted, sharply localized evoked response
in the ipsilateral cerebral cortex. All relay nuclei and association
nuclei are specific nuclei. Focal electrical stimulation of a nonspecific nucleus produces widespread activity in the cortex of
both hemispheres, at a significantly longer time delay than with
stimulation of a specific nucleus. It is thought that nonspecific nuclei play a role in modulating the excitability of large regions of
cortex. Nonspecific nuclei include the midline nuclear group, the
intralaminar nuclear group (such as the centromedian nucleus),
and a portion of the VA.
Internal Capsule
Axons pass between the diencephalon, particularly the dorsal thalamus, and the cerebral cortex in a fan-shaped mass of
fibers, the internal capsule, that courses from the central core
of the hemisphere into the brainstem.
Even though this structure consists mostly of axons that reciprocally link the thalamus and cerebral cortex, it also contains cortical efferent fibers that project to the brainstem (corticorubral,
corticoreticular, corticonuclear-corticobulbar) or spinal cord
(corticospinal).
The internal capsule has important relationship with the thalamus. As seen in axial section, the internal capsule consists of an anterior limb, genu, posterior limb,
and retrolenticular limb. The genu is located immediately lateral
to the anterior thalamic nucleus, at about the same level as the
interventricular foramen. The anterior limb extends rostrolateral from the genu and is insinuated between the caudate and
lenticular nuclei. The posterior limb extends caudolateral from
the genu and separates the thalamus from the globus pallidus.
As its name implies, the retrolenticular limb is the white matter located immediately caudal to the lenticular nucleus (Latin retro-, for "behind").
Hypothalamus
Unlike the thalamus, which is primarily related to somatic functions, the hypothalamus is mainly
involved in visceromotor, viscerosensory, and endocrine activities. The hypothalamus and related limbic structures receive
sensory input regarding the internal environment and in turn
regulate the motor systems that modify the internal environment through four mechanisms. First, the hypothalamus is
a principal modulator of autonomic nervous system function.
Second, it is a viscerosensory transducer, containing neurons
with specialized receptors capable of responding to changes in
the temperature or osmolality of blood as well as to specific
hormonal levels in the general circulation. Third, the hypothalamus regulates the activity of the anterior pituitary through the
production of releasing factors (hormone-releasing hormones).
Fourth, it performs an endocrine function by producing and
releasing oxytocin and vasopressin into the general circulation
within the posterior pituitary.
The hypothalamus can be divided into lateral, medial, and
periventricular zones. The lateral zone,
often called the lateral hypothalamic area, extends the full
rostrocaudal length of the hypothalamus and is separated from
the medial zone by a line drawn through the fornix in the sagittal plane. The medial zone is divided from rostral
to caudal into three regions: the chiasmatic, the tuberal, and the
mammillary regions. The periventricular
zone includes the neurons that border the ependymal surfaces of
the third ventricle.
Lateral Hypothalamic Zone
The lateral hypothalamic area is composed of diffuse clusters of neurons intermingled with longitudinally oriented axon bundles. The latter, which form the medial forebrain
bundle, are diffusely organized in the human brain. No discrete
named nuclei are present in this lateral area, although the supraoptic nucleus is considered by some authorities to be part of it.
Cells of the lateral hypothalamic area are involved in cardiovascular function and in the regulation of food and water intake.
Medial Hypothalamic Zone
The medial hypothalamic zone contains discrete groups of neurons whose function and connections are established. Within the chiasmatic (anterior) region are five nuclei: the preoptic,
supraoptic, paraventricular, anterior, and suprachiasmatic nuclei. Nuclei in the chiasmatic region are generally
involved in regulating hormone release (preoptic, supraoptic, periventricular), cardiovascular function (anterior), circadian
rhythms (suprachiasmatic), and body temperature and heat loss
mechanisms (preoptic). In the tuberal region are the dorsomedial, ventromedial, and arcuate nuclei. The ventromedial nucleus is regarded as the food intake (satiety) center.
Bilateral lesions of this hypothalamic region produce hyperphagia, a greatly increased food intake with resultant obesity. Cells
of the arcuate nucleus deliver peptides to the portal vessels
and, through these channels, to the anterior pituitary. Some of these peptides are releasing factors, which cause an increase in
the secretion of specific hormones by the anterior pituitary, and
some are inhibiting factors, which inhibit the secretion of specific
hormones by the anterior pituitary.
At caudal levels, the mammillary region is composed of the
posterior nucleus and the mammillary nuclei. In
humans, the mammillary nuclei consist of a large medial nucleus
and a small lateral nucleus. Although both of these nuclei receive
input via the fornix, only the medial nucleus projects to the anterior thalamic nucleus through the mammillothalamic tract. This latter bundle traverses the internal medullary lamina as it enters
the anterior nucleus. The neurons of the
posterior nucleus are involved in activities that include elevation
of blood pressure, pupillary dilation, and shivering or body heat
conservation. The mammillary nuclei are involved in the control
of various reflexes associated with feeding as well as in mechanisms relating to memory formation.
Afferent Fiber Systems
Although many axonal systems extend into the hypothalamus,
only four inputs are mentioned. The fornix
and the stria terminalis are two major afferent fiber bundles that
reach the hypothalamus. The fornix consists of axons
that largely originate in the hippocampus, and the stria terminalis
arises from neurons in the amygdaloid complex.
Fibers comprising the ventral amygdalofugal bundle exit the
amygdala and course through the substantia innominata to enter
the hypothalamus and thalamus. As already mentioned, the medial forebrain bundle passes bidirectionally through
the lateral hypothalamic region. This composite fiber bundle consists of ascending axons that originate in areas throughout the neuraxis and terminate in the hypothalamus and other axons that
exit the hypothalamus to reach forebrain and brainstem targets.
Efferent Fibers
The hypothalamus is the source of a diverse array of efferent
fibers. Several nuclei give rise to descending fibers that contribute to the dorsal longitudinal fasciculus and
the medial forebrain bundle and to diffuse projections that pass
into the tegmentum. These fiber systems project directly to
numerous brainstem nuclei as well as to preganglionic sympathetic and parasympathetic neurons in the spinal cord. Other
projections reach the thalamus and frontal cortex, and still others extend to the posterior pituitary or to the tuberohypophysial
portal system for delivery of substances to the anterior pituitary.
Ventral Thalamus (Subthalamus)
The ventral thalamus (also called subthalamus) includes the
large subthalamic nucleus, the medially adjacent prerubral area
(field H of Forel), and, posteriorly, the zona incerta. As the term ventral thalamus (or subthalamus)
implies, these cell groups are located ventral (anterior) to the
large expanse of the dorsal thalamus. The subthalamic nucleus
is a lens-shaped cell group situated rostral and posterior to the
substantia nigra and immediately inferior to a distinct myelinated fiber bundle, the lenticular fasciculus. The cells of the subthalamic nucleus receive input
from motor areas of the cerebral cortex, project to the substantia nigra, and are reciprocally connected with the globus pallidus. The subthalamic nucleus can be affected by vascular lesions
involving posteromedial branches of the posterior cerebral or posterior communicating arteries, which results in a characteristic clinical condition known as hemiballismus. Patients with
this involuntary movement disorder exhibit rapid and forceful flailing movements, which usually involve the contralateral upper
extremity. These movements can be very debilitating because the
patient has no control over their initiation or duration.
The zona incerta is located superior to the subthalamic nucleus
and is separated from it by the lenticular fasciculus.
Superior to the zona incerta are the myelinated axons of the thalamic fasciculus. The zona incerta contains output neurons that
project to a variety of locations, including the cerebral cortex,
the superior colliculus, the pretectal region, and the basilar pons. Afferent projections arise from the motor cortex and as collaterals from the medial lemniscus.
The prerubral area (Forel's field H) is located just rostral
to the red nucleus and medial to the subthalamic nucleus. There are scattered neurons in this region, and traversing
the prerubral area are fibers from the lenticular fasciculus (Forel's
field H2) that enter the thalamic fasciculus (Forel's field H1).
Epithalamus
The pineal gland, habenular nuclei, and stria medullaris thalami
are the principal components of the epithalamus. The pineal gland consists of richly vascularized
connective tissue containing glial cells and pinealocytes but no
true neurons. Mammalian pinealocytes are related to the photoreceptor elements found in this gland in lower forms, such as
amphibians. In humans, however, they remain only indirectly
light sensitive and receive information concerning photic stimuli
through a multisynaptic neural circuit.
Pinealocytes have club-like processes that are apposed to
blood vessels but do not have direct synaptic contacts with central nervous system neurons. These cells synthesize melatonin
from serotonin via enzymes that are sensitive to diurnal fluctuations in light. Levels of serotonin N-acetyltransferase increase
during the night (in the absence of photic stimulation), and the synthesis of melatonin is enhanced. Exposure to light turns off
the enzymatic activity, and melatonin production is diminished.
Thus the production of melatonin by pinealocytes is rhythmic
and calibrated to the 24-hour cycle of photic input to the retina.
This is called a circadian rhythm.
Photic stimulation of pinealocytes occurs through an indirect route. Retinal ganglion cells project to the suprachiasmatic
nucleus of the hypothalamus, which in turn influences neurons of the intermediolateral cell column in the spinal cord through
descending connections. These preganglionic sympathetic neurons project to the superior cervical ganglion, which in turn innervates the pineal gland via postganglionic fibers that travel on
branches of the internal carotid artery.
Pinealocytes also produce serotonin, norepinephrine, and neuroactive peptides, such as thyrotropin-releasing hormone, which
are normally associated with the hypothalamus. These secretory
products are released into the general circulation or the cerebrospinal fluid.
Pinealomas (tumors with large numbers of pinealocytes) are
accompanied by depression of gonadal function and delayed
puberty, whereas lesions that lead to the loss of pineal cells are associated with precocious puberty. This indicates that pineal secretory products exert an inhibitory influence on gonadal formation.
The habenular nuclei are located just anterior to the pineal
gland and consist of a large lateral nucleus and a small medial
nucleus. Both nuclei contribute axons
to the habenulointerpeduncular tract (fasciculus retroflexus),
which terminates in the midbrain interpeduncular nucleus. The
stria medullaris thalami, which arches over the medial aspect
of the dorsal thalamus near the midline, conveys input to both
habenular nuclei. The habenular commissure, a small bundle of
fibers riding on the upper edge of the posterior commissure, connects the habenular regions of the two sides.
Vasculature of the Diencephalon
The diencephalon is supplied by smaller vessels that branch from
the various arteries making up the cerebral arterial circle (circle
of Willis) and by larger arteries that originate from the proximal parts of the posterior cerebral artery.
The hypothalamus and subthalamus are supplied by central (perforating or ganglionic) branches of the circle. Anterior parts of
the hypothalamus are served by central branches (anteromedial
group) arising from the anterior communicating artery and the
A1 segment of the anterior cerebral artery and from branches of the proximal part of the posterior communicating artery. Caudal
hypothalamic regions and the ventral thalamus are supplied by
branches of the posteromedial group; these branches arise from
the posterior communicating artery and the P1 segment of the
posterior cerebral artery.
Some of the branches of the posteromedial group that arise
from the P1 segment near the basilar bifurcation are called the
thalamoperforating arteries. These vessels (of which there may
be more than one on each side) penetrate deeply to supply rostral
areas of the thalamus. If these vessels are occluded during surgery in this region, as can occur, for
example, when an aneurysm of the basilar bifurcation is clipped,
the patient can be rendered permanently comatose. Slightly
more distal branches, which usually arise from the P2 segment,
are the posterior choroidal and thalamogeniculate arteries. These
arteries also supply portions of the diencephalon. A narrow portion of the caudal and medial thalamus
bordering on the third ventricle is supplied by the medial posterior choroidal artery; the thalamogeniculate branches irrigate the
caudal thalamus, including the pulvinar and the geniculate nuclei. In addition, branches of the medial
posterior choroidal artery also serve the choroid plexus of the
third ventricle.
The anterior choroidal artery originates from the cerebral
portion of the internal carotid artery and courses caudolaterally along the trajectory of the optic tract. This
vessel serves important structures in this general area. It sends
penetrating branches into the genu of the internal capsule and
into the more inferior aspect of the posterior limb of the internal
capsule. In addition, it serves the optic tract,
inferior portions of the lenticular nucleus, the choroid plexus of
the inferior horn of the lateral ventricle, much of the amygdala,
the retrolenticular limb of the internal capsule, and large parts of
the hippocampal formation. An occlusion of this vessel, an anterior choroidal artery syndrome, results in characteristic visual
and motor deficits that reflect damage to the optic tract and the
inferior portion of the posterior limb of the internal capsule.
Although the thalamus receives a blood supply largely separate from that of the internal capsule, vascular
lesions in the thalamus may extend into the internal capsule or
vice versa. Ischemic or hemorrhagic strokes in the hemisphere may result in contralateral hemiparesis in combination with
hemianesthesia. These losses correlate with damage to corticospinal and thalamocortical fibers in the internal capsule. On
the other hand, strokes involving the larger thalamic arteries,
such as the thalamogeniculate artery, may result in total or
dissociated sensory losses. These patients may subsequently
experience persistent, intense pain (thalamic pain, Dejerine-Roussy syndrome).